The number of therapeutic drugs and drugs of abuse is increasing, thus enhancing the risk of intoxication [
1]. Interview-based diagnosis of drug misuse in patients is unreliable, exhibiting a high false-negative rate of 66% [
2]. Therefore, systematic instrumental identification of drugs is essential in clinical toxicological analysis.
Immunoassay, gas chromatography mass spectrometry, liquid chromatography, and liquid chromatography mass spectrometry are among the various methods developed for drug screening [
3456]. For confirmatory testing, mass spectrometry can detect various drugs with high sensitivity and specificity; however, it is laborious, time-consuming, and requires specialist staff for interpretation of test results and high-cost equipment [
5]. The use of immunoassays as a screening method is clinically desirable because they provide rapid turnaround time and are more easily integrated into the laboratory workflow [
4]. Numerous on-site drug-testing devices have been developed [
4], and several studies have evaluated their use in the emergency department [
789].
Triage TOX Drug Screen (Alere Inc., San Diego, CA, USA), a novel, rapid toxicology screening test based on a competitive fluorescence immunoassay, has recently been introduced [
10]. This test provides preliminary qualitative results through one-step processing following specimen application to an automatic analyzer, thus ensuring objectivity by instrumental colorimetric calibration and the subsequent printing of positive or negative results, independent of operator [
10]. This method can detect 11 drugs and/or their major metabolites, including acetaminophen (APAP), amphetamine (AMP), methamphetamine (mAMP), barbiturates (BAR), benzodiazepine (BZO), cocaine (COC), methadone (MTD), opiates (OPI), phencyclidine (PCP), tetrahydrocannabinol (THC), and tricyclic antidepressants (TCA), at concentrations higher than the urine threshold. In Korea, no study has yet addressed the usefulness of the Triage TOX Drug Screen for detecting drugs of abuse and therapeutic drugs. The present study evaluated the precision of the on-site immunoassay drug screening device Triage TOX Drug Screen and compared the efficacy with ultra-performance liquid chromatography with tandem mass spectrometry (UPLC-TMS).
A total of 105 urine specimens were collected from January 2014 to April 2016 from intoxicated patients who visited the emergency center and health check-up patients in a tertiary-care hospital in Seoul, Korea. All specimens were anonymized by removing any identifiable information, including patient name, address, and hospital number, and stored at 4℃ until screened (within 3 days of collection). The specimens were combined and evaluated in batches by using the Triage TOX Drug Screen assay and UPLC-TMS on April 2016. This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (IRB 2016-005-001).
The Triage TOX Drug Screen was performed on an Alere Triage Meter Pro (Alere Inc.) automated analyzer according to the manufacturer's instructions [
11]. The urine specimen reacts with a fluorescent antibody or drug conjugates and flows through the test device by capillary action. The Triage Meter Pro Reader records fluorescence in the detection zone and interprets findings as positive or negative. The positive or negative results are displayed on the Meter screen approximately 15 min from specimen loading. All results are stored in the Meter memory for display or printed when needed. If connected, the Meter can transmit the results to the laboratory information system. UPLC-TMS was performed on a Waters Acquity UPLC system (Waters Corp., Milford, MA, USA), which can simultaneously screen up to 177 of the most prevalent medical drugs and drugs of abuse in urine; total instrumental analysis time is 17 min except for specimen preparation time [
5]. The analysis was performed in multiple reaction monitoring (MRM) mode for each compound and positive electrospray ionization using the transactions: mass to charge ratio (m/z) (MRMs available upon request).
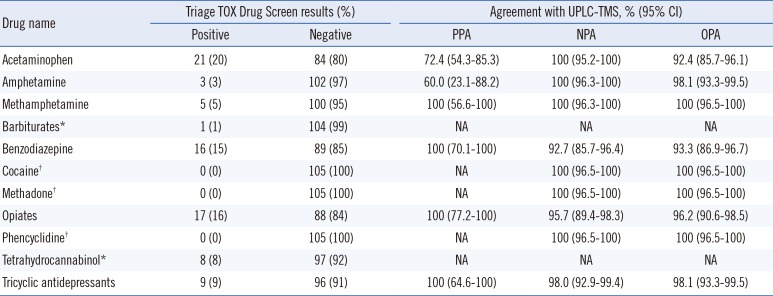
The drugs identified in the urine specimens included APAP (21/105, 20%), AMP (3/105, 3%), mAMP (5/105, 5%), BAR (1/105, 1%), BZO (16/105, 15%), OPI (17/105, 16%), THC (8/105, 8%), and TCA (9/105, 9%). All specimens were negative for COC, MTD, and PCP.
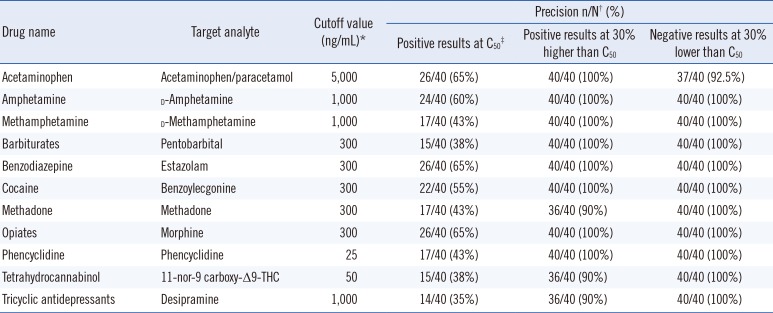
The precision was evaluated following the CLSI guidelines EP12-A2 [
12]. The cutoff values for each compound are as listed in the manufacturers' instructions [
11] (
Table 1). The cutoff values were verified using the standards provided by the manufacturers. Each standard was diluted to C
50 (the concentration yielding 50% positive results). Based on the EP12-A2 guidelines, analyte concentrations, including C
50, 30% lower than C
50 (-30%), and 30% higher than C
50 (+30%), were detected 40 times using the Triage TOX Drug Screen. The precision results of the Triage TOX Drug Screen assay are presented in
Table 1. The +30% specimens yielded 36/40 to 40/40 (90-100%) positive results and the -30% specimens yielded 37/40 to 40/40 (92.5-100%) negative results (
Table 1). It is likely that the -30% to +30% concentration range encompasses the C
5-C
95 interval, because we observed more than 36/40 (90%) positive results at the +30% specimen concentration and more than 36/40 (90%) negative results at the -30% specimen concentration.
A comparison of the Triage TOX Drug Screen and UPLC-TMS results is presented in
Table 2. The positive percent agreement of the Triage TOX Drug Screen for COC, MTC, and PCP could not be obtained because of the lack of a positive specimen within the study period; the negative percent agreement was 100% (95% CI, 96.5-100%). The overall percent agreement of the Triage TOX Drug Screen assay ranged from 92.4 to 100% for all evaluated drugs. BAR and THC were undetectable by UPLC-TMS, hence comparative analysis could not be conducted.
Ten discordant Triage TOX Drug Screen-negative and UPLC-TMS-positive results were observed for APAP and AMP (APAP, n=8; AMP, n=2). A previous study reported that the Triage TOX Drug Screen detected urine APAP with good accuracy; however, the sensitivity and specificity decreased because of low urine APAP concentration [
13]. UPLC-TMS exhibited high sensitivity for screening low concentrations of drugs in urine [
5]; thus, the discrepancies in APAP and AMP may be due to the difference in sensitivity between the methods. However, we could not confirm this hypothesis because we did not carry out the quantification test. Of the Triage TOX Drug Screen BZO-positive cases, seven urine specimens were negative by UPLC-TMS. According to the manufacturer's instructions [
11], the Triage TOX Drug Screen can detect metabolites and other analogs, such as alpha-OH-alprazolam glucuronide, urine metabolites of alprazolam, and flurazepam metabolites, which cannot be detected by the UPLC-TMS system [
5]. The results for the other drugs, including OPI and TCA, were Triage TOX Drug Screen-positive but UPLC-TMS-negative (OPI, n=4; TCA, n=2). The present study did not include COC-, MTD-, and PCP-positive urine specimens; however, there were no discordant cases with UPLC-TMS.
The results of the present study indicate that the performance of the Triage TOX Drug Screen is comparable to UPLC-TMS, with an overall percent agreement of 92.4-100%. The Triage TOX Drug Screen has a shorter total process time compared with UPLC-TMS (15 min vs 24 hr). In addition, it offers the advantage of a one-step method and an instrument-read cartridge that bypasses the need for visually interpreting bands. Previous studies evaluating the performance of Triage on-site drug-testing have also shown good results for screening drugs of abuse and therapeutic drugs [
1013].
This study had some limitations. First, we could not perform quantification of the drugs included in the Triage TOX Drug Screen. Although some level of a drug may be present in a urine specimen, the specimen would still be considered negative if the level was below the cutoff concentration. In addition, BAR and THC were included only in the Triage TOX Drug Screen panel, so one BAR-positive and eight THC-positive specimens could not be compared with the UPLC-TMS results.
Collectively, the Triage TOX Drug Screen showed adequate performance in terms of cutoff verification, precision, and the comparison test. The advantages include easy accessibility, short analysis time, and objective results. This assay could constitute a useful screening method for drugs of abuse and therapeutic drugs in urine.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download