Dear Editor,
Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome is a rare, autosomal dominant, primary immunodeficiency disorder [1], with only 65 cases reported worldwide [2]. This disorder is caused by a gain-of-function mutation in CXCR4, which, along with its ligand CXCL12 (stromal cell-derived factor 1 [SDF-1]), plays a role in retention of mature neutrophils in the bone marrow (BM) [3]. Here we describe a Korean child with WHIM syndrome who had a novel CXCR4 mutation. To the best of our knowledge, this is the first report of WHIM syndrome in Korea.
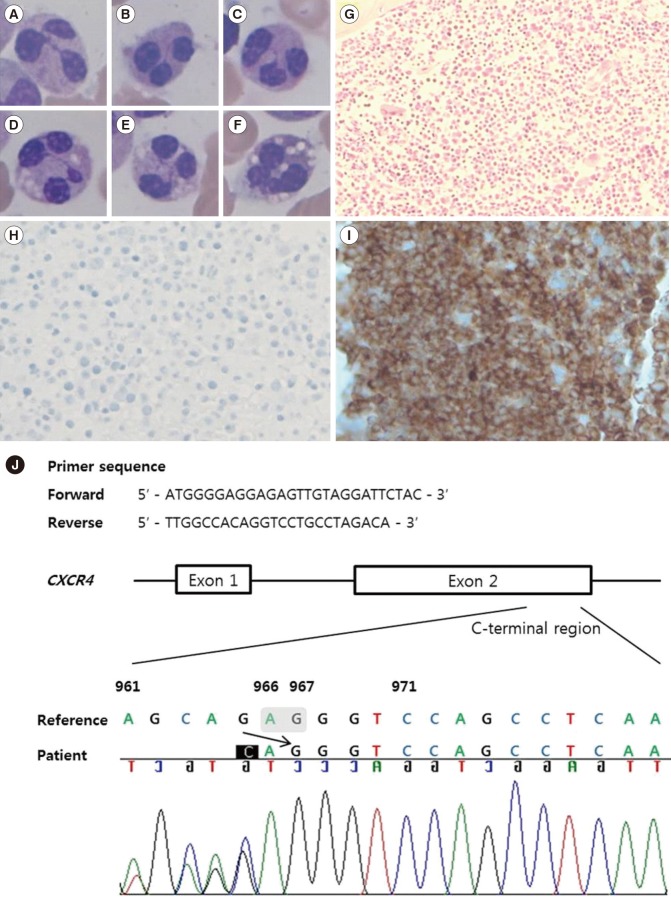
A Korean male was born at full term (3.9 kg) without perinatal problems. There was no family history of susceptibility to infection or neutropenia. At one month of age, he was brought to the Department of Plastic Surgery for correction of cleft lip, and complete blood count (CBC) revealed white blood cell counts of 2.09 ×109/L (absolute neutrophil count [ANC] 0/L), hemoglobin 99 g/L, and platelets 313×109/L. Differential count showed 98% lymphocytes and 2% monocytes, with absence of neutrophils on the peripheral blood smear. The surgery was postponed, and he was referred to a pediatric hematology specialist. At one year and five months of age, he visited the emergency department because of a right inguinal hernia; ANC was 0.06×109/L, and the surgery was performed successfully after administration of granulocyte-colony stimulating factor (G-CSF) (Fig. 1). At one year and eight months of age, BM examination showed a hypercellular BM with mild granulocytic hyperplasia (myeloid:erythroid ratio, 7:1) and right-shifted maturation. He was hospitalized for pneumonia and acute gastroenteritis at two years and six months of age. At two years and eight months of age, BM re-examination revealed plentiful granulopoietic cells with right-shifted maturation, and neutrophils showed characteristics of dysplasia such as pyknotic lobes with long filaments. Multifocal collections of mature neutrophils (myelokathexis) were observed in a BM section (Fig. 2A-I). The IgG level was decreased to 2.82 g/L (reference range: 7.0–17.0 g/L), IgA level was 0.19 g/L (0.9–4.0 g/L), and IgM level was 0.59 g/L (0.45–2.3 g/L), but warts were absent. Sanger sequencing of CXCR4 from BM aspirates revealed the novel sequence variant c.966_967delAG (p.Gly323Valfs*20) (Fig. 2J). Although he had no definite warts, the collective evidence of hypogammaglobulinemia, myelokathexis, multiple histories of infections, and CXCR4 mutation confirmed the diagnosis of WHIM syndrome.
Congenital neutropenia involving mutation of ELANE, CSF3R, WAS, and HAX1 is characterized by maturation arrest of granulocytic-series cells [4]. In contrast to maturation arrest, CXCR4 mutation causes retention of mature neutrophils in the BM, resulting in peripheral neutropenia. The fate of retained neutrophils due to enhanced CXCR4 activity delays the release of mature neutrophils from the BM, resulting in neutropenia and senescence with apoptosis of mature neutrophils retained in the BM [5]. Since CXCR4 signaling also regulates the expression of CD20 on B cells, consequential hypogammaglobulinemia develops in WHIM [6].
The variant found in this case, c.966_967delAG (p.Gly323Valfs *20), is a novel pathogenic variant. The most common CXCR4 mutation among WHIM syndrome patients is p.Arg334*, and other reported mutations are p.Gly336*, p.Ser338*, p.Glu343*, p.Ser339fs, p.Lys329fs, and p.Ser324fs (the mutation most similar to that in our case) [37]. CXCR4 has an extracellular N-terminus encoding transmembrane helices, and a C-terminus encoding the cytoplasmic portion. The disulfide bond in the N-terminus is essential for CXCL12 binding, and G-CSF induces cleavage of the N-terminus of CXCR4 on hematopoietic stem cells, reducing the retention of hematopoietic cells in the BM [8]. All of the CXCR4 mutations associated with WHIM syndrome reside in the C-terminus, resulting in truncation of the C-terminal domain of the protein, with persistent CXCR4 activation and BM myeloid cell trafficking [1]. Immunohistochemical staining of a BM section with anti-CXCR4 antibody showed absence of CXCR4 surface protein owing to truncation of the CXCR4 C-terminal proportion in this patient (Fig. 2H). Somatic mutations in CXCR4 were identified in 30% of Waldenström macroglobulinemia (WM) patients, in whom the locations of somatic mutations are similar to those in WHIM syndrome [9]. Although the most well-known cause of WHIM syndrome is CXCR4 mutation, WHIM patients without CXCR4 mutation showing hyperresponsiveness to CXCL12 have been reported [10].
Among the 37 WHIM syndrome patients, the frequency of warts, hypogammaglobulinemia, and neutropenia as a first presentation was 78.6%, 89.6%, and 91.7%, respectively [3]. Considering that patients with WHIM syndrome typically present numerous warts on the hands, feet, and trunk, with variable age at diagnosis, the current patient is atypical in that he had no warts and the initial BM features showed no apparent dysplastic neutrophils and myelokathexis. More to the point, these atypical features can lead to missing the diagnosis. In this case, we performed the follow-up BM examination and CXCR4 sequencing and finally diagnosed a WHIM syndrome with a novel CXCR4 mutation. To avoid missing the diagnosis, suspicion of WHIM is important and follow-up BM examination should be considered.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2014R1A2A1A10052286).
References
1. Pozzobon T, Goldoni G, Viola A, Molon B. CXCR4 signaling in health and disease. Immunol Lett. 2016; 177:6–15. PMID: 27363619.
2. Ana Rath, Stéphanie Nguengang Wakap, Samuel Demarest, Valérie Lanneau (Eds), Prevalence of rare diseases: Bibliographic data, Orphanet Report Series, Rare Diseases collection, November 2016, Number 1 : Diseases listed in alphabetical order. Updated on Nov 2016. http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_diseases.pdf.
3. Kawai T, Malech HL. WHIM Syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009; 16:20–26. PMID: 19057201.
4. Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot CB. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis. 2011; 6:26. PMID: 21595885.
5. Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007; 13:587–596. PMID: 17435771.
6. Pavlasova G, Borsky M, Seda V, Cerna K, Osickova J, Doubek M, et al. Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood. 2016; 128:1609–1613. PMID: 27480113.
7. Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis. 2012; 7:71. PMID: 23009155.
8. Pawig L, Klasen C, Weber C, Bernhagen J, Noels H. Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol. 2015; 6:429. PMID: 26347749.
9. Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014; 123:1637–1646. PMID: 24366360.
10. Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005; 105:2449–2457. PMID: 15536153.
Fig. 1
WBC and ANC results according to the patient's age and clinical events.
Abbreviations: CBC, complete blood count; WBC, white blood cell; ANC, absolute neutrophil count; G-CSF, granulocyte colony stimulating factor; BM, bone marrow; y, year(s); m, month(s).
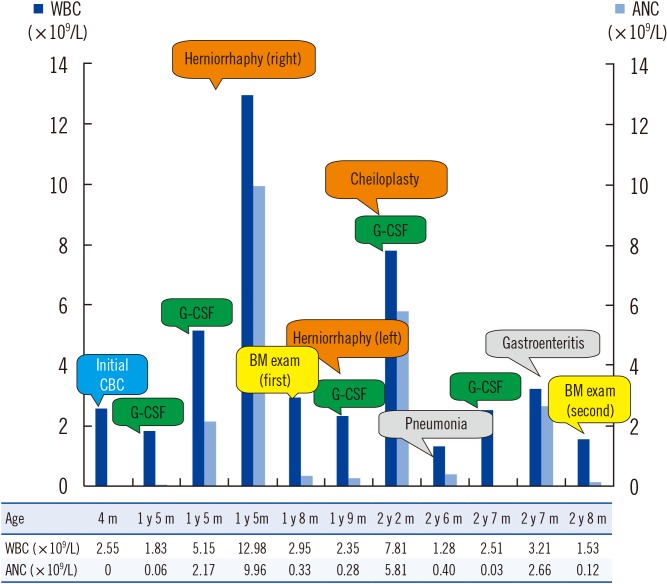
Fig. 2
Results of bone marrow examination and Sanger sequencing of the CXCR4 gene. (A–F) Neutrophils with pyknotic nuclei, lobes separated by long strands of chromatin, and cytoplasmic vacuoles were observed (Wright Giemsa stain, ×1,000); (G) Marrow with hypercellularity and multifocal collections of mature neutrophils were observed (hematoxylin and eosin stain, ×200); (H) Anti-CXCR4 immunohistochemical stain showed an absence of CXCR4 surface protein; (I) A mouse spleen section was stained as a positive control for anti-CXCR4 stain (×400; Ab1670, Abcam, Cambridge, UK); (J) Chromatogram was obtained from Sanger sequencing of the CXCR4 gene (reference sequence: NM_003467.2). Reverse sequence reading from the 3′ end revealed the c.966_967delAG frameshift variant. Designation was based on the 3′ rule that the most 3′ position possible of the reference sequence is arbitrarily assigned to have been changed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download