Dear Editor,
Almost all cases of CML show typical clinical features such as marked leukocytosis in the peripheral blood (PB), splenomegaly, and granulocytic hyperplasia in the PB and bone marrow (BM). CML without leukocytosis in the PB is rare but could occur if “undiagnosed” CML patients received cytoreductive therapy due to the patient's predisposing hematologic malignancies. In contrast, the presence of the BCR-ABL1 mutation without the clinical features of CML and unrelated to cytoreductive therapy (so-called “preleukemic phase” or “smoldering” CML) is very rare [123]. Here, we report a rare case of hidden preleukemic phase of CML presenting without leukocytosis in the PB unrelated to prior chemotherapy in a diffuse large B cell lymphoma (DLBCL) patient. To our knowledge, this is the first case of preleukemic phase of CML in the Korean population.
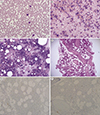
A 67-yr-old man was admitted owing to abdominal pain in April 2016. At admission, he exhibited multiple lymphadenopathy and was diagnosed as having DLBCL after right supraclavicular lymph node biopsy. His hemogram results at admission were as follows: white blood cell count, 8.98×109/L; hemoglobin, 15.2 ×10 g/L; platelets, 2.04×1012/L; segmented neutrophils, 71.7%; lymphocytes, 10.5%; monocytes, 12.6%; eosinophils, 1.0%; and basophils, 4.2%. PB smear showed normal granulocytic maturation pattern without atypical lymphocytes or immature granulocytes (Fig. 1A). The patient's BM aspirates and touch print results showed slightly hypercellular marrow with mild granulocytic hyperplasia (myeloid:erythroid ratio=6.93:1, and differential counts as follows: myeloblasts, 2.0%; promyelocytes, 2.4%; myelocytes, 14.8%; metamyelocytes, 14.8%; band neutrophils, 15.9%; segmented neutrophils, 19.3%; eosinophils, 2.4%; and basophils, 0.0%) without atypical lymphocytes (Fig. 1B and 1C). The BM biopsy section showed 60% cellularity with mild granulocytic hyperplasia, eosinophilia, and megakaryocytic hyperplasia (4.5/high power field) without evidence of neoplastic lymphoid cell infiltration (Fig. 1D), demonstrated by the CD45RO and CD20 immunohistochemical stain results (Fig. 1E and 1F). Based on these findings, the initial diagnosis was given as (1) no evidence of BM involvement of DLBCL, and (2) mild granulocytic and megakaryocytic hyperplasia, suggestive of reactive changes. However, karyotyping results were 46,XY,t(9;22)(q34;q11.2)[19]/46,XY[1] (Fig. 2) and major BCR/ABL1 reverse transcriptase PCR demonstrated the presence of major BCR/ABL1 fusion transcript. From these results, the final diagnosis was revised as preleukemic phase of CML without leukocytosis in the PB, unrelated to prior chemotherapy associated with initial diagnosis of DLBCL. After the final diagnosis, the patient received R-CHOP chemotherapy plus imatinib to manage both DLBCL and preleukemic phase of CML. In the follow-up examinations performed six months after diagnosis, the patient's karyotype returned to normal and at present, the patient is scheduled to undergo 12 months follow-up examinations after diagnosis.
Although previous studies have reported a latency period of several years between the detection of the t(9;22)(q34;q11.2) and the development of CML clinical features [123], hidden preleukemic phase of CML in patients without a history of cytoreductive therapy is still considered rare. CML without leukocytosis in the PB can occur if true CML patients received cytoreductive therapy related to other hematologic malignancies, but these patients should not be regarded as preleukemic phase of CML because they would have typical CML symptoms. A recent study tried to identify clues that may be helpful in detecting preleukemic phase of CML in asymptomatic patients and reported the presence of peripheral basophilia (57% of cases), and increased number of small, hypolobulated megakaryocytes in BM, which may distinguish preleukemic CML from other leukemoid reactions [3]. Our present case also showed peripheral basophilia and mild megakaryocytic hyperplasia with small-sized, hypolobulated forms in BM biopsy, which would support results from the previous study [3]. A likely explanation for the preleukemic phase of CML is that BCR-ABL1 occurs in multipotent stem cells and is considered an oncogenic driver mutation. BCR-ABL1 confers a growth advantage over normal cells, and massive expansion of BCR-ABL1-positive cells facilitates the acquisition of additional mutations leading to the development of overt CML [3].
Currently, there are no guidelines for the treatment of patients with preleukemic phase of CML. Since the development of preleukemic CML cells into overt leukemia would be associated with an increase in BCR-ABL1 levels, tyrosine kinase inhibitor (TKI) therapy may delay the transformation to overt CML in these patients, and this hypothesis is supported by a previous study [3]. Our patient was also treated with TKI and the effect was successful. More follow-up results would add some clues about the efficacy of TKI treatment in cases of preleukemic CML.
Figures and Tables
Fig. 1
Peripheral blood smear, bone marrow aspiration, touch print, biopsy, and immunohistochemical staining results of the patient at initial diagnosis. (A) Peripheral blood smear results of the patient demonstrating the absence of leukocytosis, atypical lymphocytes or immature granulocytes (400×, Wright stain). (B, C) Bone marrow aspiration smear and touch print indicating slightly hypercellular marrow with mild granulocytic and megakaryocytic hyperplasia without atypical lymphocytes (B, 200×; C, 400×; Wright stain). (D) Bone marrow biopsy section demonstrating approximately 60% cellularity with mild granulocytic hyperplasia, eosinophilia, and megakaryocytic hyperplasia without evidence of neoplastic lymphoid cell infiltration (40×, hematoxylin & eosin stain). (E, F) Immunohistochemical stain results of CD45RO (200×) and CD20 (200×) demonstrating the infiltration of a few reactive T and B lymphocytes, respectively.
References
1. Hudnall SD, Northup J, Panova N, Suleman K, Velagaleti G. Prolonged preleukemic phase of chronic myelogenous leukemia. Exp Mol Pathol. 2007; 83:484–489.

2. Bennett JM, Dsouza KG, Patel M, O'Dwyer K. “Preleukemic or smoldering” chronic myelogenous leukemia (CML): BCR-ABL1 positive: A brief case report. Leuk Res Rep. 2014; 4:12–14.
3. Aye le L, Loghavi S, Young KH, Siddiqi I, Yin CC, Routbort MJ, et al. Preleukemic phase of chronic myelogenous leukemia: morphologic and immunohistochemical characterization of 7 cases. Ann Diagn Pathol. 2016; 21:53–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download