Identification of
viridans streptococci at the species level is difficult because of the high degree of genotypic and phenotypic similarity of some species. Unfortunately, phenotypic test systems that are widely used in clinical laboratory, like Rapid ID32 Strep (bioMérieux, Lyon, France) and VITEK 2 (bioMérieux), do not always accurately identify some species in this heterogeneous bacterial group [
123]. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS)-based methodologies seem to have great potential for reliably identifying
viridans streptococci in clinical laboratories [
45].
In this study, two MALDI-TOF-MS-based approaches were compared for their ability to identify viridans streptococci isolates.
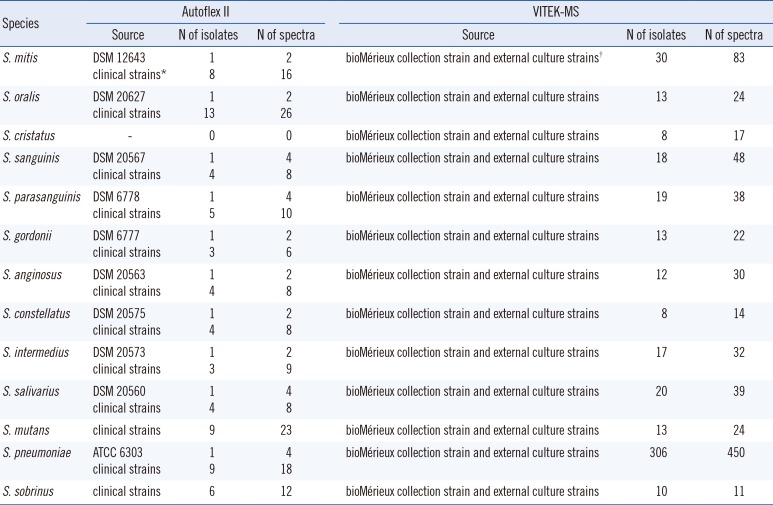
Clinical strains of viridans streptococci (n=184) were used in this study. All isolates were previously biochemically identified as streptococci by using the Rapid ID32 Strep System (bioMérieux) and then stored at −80℃ in CRYOBANK BLUE tubes from Mast Diagnostica (Reinfeld, Germany). For analysis, the strains were cultured on Columbia Blood Agar (Thermo Fisher Scientific, Oxoid Microbiology Products, Hampshire, UK) at 36–37℃with 5% CO2 for 18–24 hr.
A reference database comprising a well-characterized spectrum of reference and clinical strains of
Streptococcus mitis,
S. oralis,
S. pneumoniae,
S. intermedius,
S. salivarius,
S. sanguinis,
S. parasanguinis,
S. gordonii,
S. constellatus,
S. anginosus,
S. mutans, and
S. sobrinus was developed in-house (
Table 1). For this, individual colonies of streptococcal isolates were recultured in brain-heart infusion broth overnight and prepared by using the extraction methods described by Friedrichs et al [
4]. Mass spectra were acquired by using an Autoflex II MALDI-TOF mass spectrometer (Bruker Daltonics GmbH, Bremen, Germany) with a nitrogen laser (337 nm) operating in positive linear mode (delay 150 nsec, voltage 20 kV, mass range 2–20 kDa) using FLEXCONTROL software version 2.4 (Bruker Daltonics). The spectra were externally calibrated by using the standard calibrant mixture, Protein Calibration Standard I (Bruker Daltonics). Automated peak extraction from data files of the reading was performed after transferring in FLEXANALYSIS software version 2.4 (Bruker Daltonics). To identify bacterial species, similarity analysis between peak lists was performed by using a hierarchical clustering procedure in MatLab software (Version 7.10.0.499; The Math-Works Inc., Natick, MA, USA), as described by Friedrichs et al [
4].
Table 1
Details of the in-house database used by Autoflex II and V2.0 Knowledge Base used with VITEK-MS
|
Species |
Autoflex II |
VITEK-MS |
|
Source |
N of isolates |
N of spectra |
Source |
N of isolates |
N of spectra |
|
S. mitis
|
DSM 12643 |
1 |
2 |
bioMérieux collection strain and external culture strains†
|
30 |
83 |
|
clinical strains*
|
8 |
16 |
|
S. oralis
|
DSM 20627 |
1 |
2 |
bioMérieux collection strain and external culture strains |
13 |
24 |
|
clinical strains |
13 |
26 |
|
S. cristatus
|
- |
0 |
0 |
bioMérieux collection strain and external culture strains |
8 |
17 |
|
S. sanguinis
|
DSM 20567 |
1 |
4 |
bioMérieux collection strain and external culture strains |
18 |
48 |
|
clinical strains |
4 |
8 |
|
S. parasanguinis
|
DSM 6778 |
1 |
4 |
bioMérieux collection strain and external culture strains |
19 |
38 |
|
clinical strains |
5 |
10 |
|
S. gordonii
|
DSM 6777 |
1 |
2 |
bioMérieux collection strain and external culture strains |
13 |
22 |
|
clinical strains |
3 |
6 |
|
S. anginosus
|
DSM 20563 |
1 |
2 |
bioMérieux collection strain and external culture strains |
12 |
30 |
|
clinical strains |
4 |
8 |
|
S. constellatus
|
DSM 20575 |
1 |
2 |
bioMérieux collection strain and external culture strains |
8 |
14 |
|
clinical strains |
4 |
8 |
|
S. intermedius
|
DSM 20573 |
1 |
2 |
bioMérieux collection strain and external culture strains |
17 |
32 |
|
clinical strains |
3 |
9 |
|
S. salivarius
|
DSM 20560 |
1 |
4 |
bioMérieux collection strain and external culture strains |
20 |
39 |
|
clinical strains |
4 |
8 |
|
S. mutans
|
clinical strains |
9 |
23 |
bioMérieux collection strain and external culture strains |
13 |
24 |
|
S. pneumoniae
|
ATCC 6303 |
1 |
4 |
bioMérieux collection strain and external culture strains |
306 |
450 |
|
clinical strains |
9 |
18 |
|
S. sobrinus
|
clinical strains |
6 |
12 |
bioMérieux collection strain and external culture strains |
10 |
11 |

VITEK MS (bioMérieux) was operated on the V2.0 Knowledge Base for clinical use and employed for identification a direct colony method. Colonies were picked from Columbia blood agar plates and spotted onto the polymeric target slides (bioMérieux) in duplicate. After air drying, 1 µL matrix solution (α-cyano-4-hydroxycinnamic acid, VITEK MS CHCA) was added. The target slides were then loaded into the mass spectrometer (VITEK MS). The spectra were compared to the VITEK MS V2.0 Knowledge Base for clinical use. Escherichia coli ATCC 8739 was used to calibrate and control the method according to the manufacturer's instructions. Successful identification was considered only at 99.9% probability. For the unidentified or misidentified strains, we repeated the protocol once. Since the V2.0 Knowledge Base for clinical use cannot differentiate between S. mitis and S. oralis, S. mitis/S. oralis was considered as a match for species identification.
Discrepant results between the two systems (including the unidentified strains) were resolved by 16S rRNA gene sequencing. MALDI-TOF MS results discordant with 16S rRNA gene sequencing were considered incorrect.
Chi-square test was used to compare species-level identification percentages.
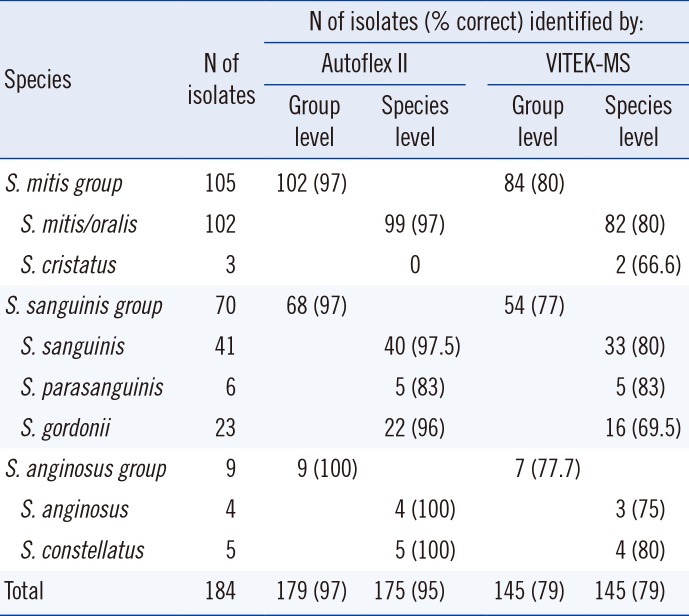
The Autoflex II-based method correctly identified 179 (97%) isolates to the group level and 175 (95%) isolates to the species level. Five strains were incorrectly identified to the group level. Two strains belonging to the
S. sanguinis group were misidentified as members of the
S. mitis group, and three members of the
S. mitis group were misidentified as members of the
S. sanguinis group. In comparison, VITEK-MS correctly identified 145 (79%) isolates to the group and species levels (
Table 2). Eight strains were misidentified, and 31 remained repeatedly unidentified. Four strains belonging to the
S. sanguinis group were misidentified as members of the
S. mitis group, and 4 strains belonging to the
S. mitis group were misidentified as members of the
S. sanguinis group. The 31 unidentified strains were as follows: 17 members of the
S. mitis group, 12 members of the
S. sanguinis group, and two members of the
S. anginosus group. The difference between the two methods was statistically significant (
P<0.05) for both group and species levels.
Table 2
Comparison of identification results for 184 strains of viridans streptococci obtained with the Autoflex II-based method or with the VITEK-MS-based method
|
Species |
N of isolates |
N of isolates (% correct) identified by: |
|
Autoflex II |
VITEK-MS |
|
Group level |
Species level |
Group level |
Species level |
|
S. mitis group
|
105 |
102 (97) |
|
84 (80) |
|
|
S. mitis/oralis
|
102 |
99 (97) |
82 (80) |
|
S. cristatus
|
3 |
0 |
2 (66.6) |
|
S. sanguinis group
|
70 |
68 (97) |
|
54 (77) |
|
|
S. sanguinis
|
41 |
40 (97.5) |
33 (80) |
|
S. parasanguinis
|
6 |
5 (83) |
5 (83) |
|
S. gordonii
|
23 |
22 (96) |
16 (69.5) |
|
S. anginosus group
|
9 |
9 (100) |
|
7 (77.7) |
|
|
S. anginosus
|
4 |
4 (100) |
3 (75) |
|
S. constellatus
|
5 |
5 (100) |
4 (80) |
|
Total |
184 |
179 (97) |
175 (95) |
145 (79) |
145 (79) |

The taxonomic changes over the past several years, poor capacity of Rapid ID 32 Strep and VITEK 2 systems to differentiate them, and their genetic and proteomic homology have made the identification of
viridans streptococci challenging in clinical laboratories [
26].
In this study, two MALDI-TOF-MS-based methods were compared for their ability to identify viridans streptococci. Three different groups and eight different species were represented in this study: S. mitis group (n=105), S. anginosus group (n=9), and S. sanguinis group (n=70).
The Autoflex II-based in-house identification method showed superior performance to the commercial VITEK-MS system at both group and species levels. Its database did not include Streptococcus cristatus, so consequently all 3 S. cristatus strains were misidentified as S. oralis. The VITEK-MS system, which includes S. cristatus in its database, correctly identified two of these three strains, with only one strain being misidentified as S. parasanguinis.
Our in-house method misidentified three strains belonging to the S. mitis group as S. gordonii (n=2) and S. sanguinis (n=1). Furthermore, two strains belonging to the S. sanguinis group were misidentified as members of the S. mitis group. One misidentification at the species level occurred within the S. sanguinis group: an S. parasanguinis strain was misidentified as S. gordonii.
VITEK-MS failed repeatedly to identify 31 strains (17%), 17 (16%) of them belonging to the S. mitis group, 12 (17%) strains from the S. sanguinis group, and two (22%) strains as members of the S. anginosus group. Whenever VITEK-MS correctly identified a strain at the group level, the species identification was also correct. Four different strains of the S. sanguinis group (two strains of S. sanguinis, one strain each of S. gordonii and S. parasanguinis) were misidentified as S. mitis/oralis. Additional four strains belonging to the S. mitis group were misidentified as members of the S. sanguinis group (two S. gordonii strains and two S. parasanguinis strains).
Both systems misidentified strains belonging to all groups; hence, we cannot conclude that one specific group of streptococci proved to be more difficult to differentiate than another.
While the Autoflex II-based method identified all strains, correctly or not, VITEK-MS failed to identify 17% of strains. The unidentified strains were members of all tested groups.
Another study compared the performance of two commercially available systems: VITEK-MS (bioMérieux) and MALDI Biotyper (Bruker Daltonics) using 54 strains and showed that both systems performed better than the commercially available biochemical methods [
7]. Overall, the MALDI Biotyper and VITEK-MS systems gave a correct species-level identification in 94% and 69% of strains, respectively. In our study using more strains (n=184), we observed better performance of VITEK-MS, with 75% of strains being correctly identified. Rychert et al [
8] and Dubois et al [
9] reported an even better performance of VITEK-MS with 82% of strains being correctly identified from 218 streptococcal isolates and 90.8% being correctly identified from 335
viridans streptococci strains. It is not straightforward to compare these results with the performance of the Autoflex II-based method used in this study, because it is not a commercial system but uses cell extracts instead of whole cells and in-house developed software and database. When compared with the results by Friedrichs et al [
4] that used the same system, our results were similar.
A combination of cell extraction, spectra acquisition using the Autoflex II, in-house developed software, and our own database seemed to more reliably identify viridans streptococci strains than the VITEK-MS system. The combination of an extraction method with a powerful analysis software and a database containing carefully chosen, well-identified strains can provide a useful tool for identifying viridans streptococci species.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download