Abstract
Background
The emergence of fosfomycin resistance and extended-spectrum β-lactamase (ESBL) genes is a serious threat to public health and a new challenge in shigellosis treatment. The purpose of this study was to identify fosfomycin resistance and characterize β-lactamase genes in fos-carrying isolates of Shigella flexneri from patients in China.
Methods
A total of 263 S. flexneri isolates were collected from 34 hospitals in the Anhui Province of China during September 2012-September 2015 and screened for fosA3, fosA, and fosC2 by PCR amplification and sequencing. The fos-carrying isolates were then screened for β-lactamase genes. The clonal relationships between fosA3-carrying isolates, the transmissibility of fosfomycin resistance, replicon types of plasmids carrying fosfomycin resistance genes and other associated resistance genes were investigated.
Results
Twenty-five of the 263 isolates (9.5%) showed resistance to fosfomycin, and 18 (6.8%) were positive for fosA3. None of the isolates was positive for fosA or fosC2. Seventeen of the isolates carrying fosA3 (94%) were CTX-M producers (seven CTX-M-55, five CTX-M-14, and five CTX-M-123), while three (16.7%) were TEM producers (TEM-1).Sixteen (88.9%) fosA3-carrying isolates exhibited multi-drug resistance. The replicon types of the 13 fosA3-carrying plasmids were IncF (n=13), IncHI2 (n=3), IncIl-Ir (n=2), and IncN (n=1).
Conclusions
Our results indicated that fosA3 could spread through plasmids in S. flexneri isolates, along with the blaCTX-M and blaTEM, which facilitate its quick dispersal. To the best of our knowledge, this is the first report of CTX-M-123-type ESBLs in S. flexneri isolates from patients in China.
Shigellosis is still a major cause of morbidity and mortality worldwide, especially in developing countries, where 99% of the estimated 165 million annual cases occur. Children under five years of age are involved in more than a half of the cases and deaths [1]. In China, shigellosis is the top-ranked among gastrointestinal infectious illness with respect to the incidence. The predominant pathogen responsible for shigellosis is Shigella flexneri [2]. In recent decades, antimicrobial agents have been effective in alleviating the dysenteric syndrome associated with shigellosis. The growing prevalence of extended-spectrum β-lactamase (ESBL)-producing S. flexneri isolates in many countries has rekindled interest in fosfomycin (FOM) as a therapeutic agent [34]. FOM is a naturally occurring antibacterial agent with a broad spectrum of action against both gram-positive and gram-negative bacteria. Despite its worldwide use in clinical practice for nearly four decades, FOM has remained effective against common uropathogens and has not given rise to clinically relevant resistant strains [345]. However, a FOM resistance gene, fosA3, was reported in Escherichia coli [6]. This gene was also detected in CTX-M-producing and multidrug-resistant E. coli and Klebsiella pneumoniae isolates [678910]. It has been suggested that the increasing prevalence of fosA3 was due to the dissemination of IncI and IncN plasmids, rather than the clonal expansion of specific strains. The purpose of this study was to identify FOM resistance and to characterize β-lactamase genes in fos-carrying S. flexneri isolates obtained from patients in China.
A total of 263 non-duplicate S. flexneri isolates were collected from 34 secondary level hospitals in the Anhui Province of China, between September 2012 and September 2015. The ages of the subjects ranged from six months to 78 yr. Individual isolates were identified by using standard microbiological and biochemical methods. All Shigella isolates were confirmed by using the API-20E system (bioMérieux, Marcy l' Étoile, France) and serotyped by using commercial antisera (Denka Seiken Co. Ltd., Tokyo, Japan). E. coli ATCC 25922, K. pneumoniae ATCC 700603, and sodium azide-resistant E. coli J53 were stored at the Anhui Center for Surveillance of Bacterial Resistance (Hefei, Anhui, China).
The study was conducted in accordance with the guidelines of Declaration of Helsinki, the principles of Good Clinical Practice, and Chinese regulatory requirements, and was approved by the local Ethics Committees of the First Affiliated Hospital of Anhui Medical University (Hefei, China). All patients gave written informed consent.
All the isolates were screened for fosA3, fosA, and fosC2 genes by using methods described previously [11]. The fos-carrying isolates were screened for β-lactamase genes by using primer pairs described previously [12]. All the purified PCR products were sequenced on an ABI Prism 3730 sequencer (Applied Biosystems, Foster City, CA, USA). Sequence alignment was performed with the GenBank nucleotide database, using the nucleotide BLAST program.
The minimum inhibitory concentrations (MICs) of the following 11 antimicrobial agents were determined by using the agar dilution method, according to the CLSI guidelines (2015): FOM, cefotaxime (CTX), ceftriaxone (CRO), ceftazidime (CAZ), chloramphenicol (CHL), piperacillin-tazobactam (PTZ), ciprofloxacin (CIP), levofloxacin (LEV), amikacin (AMK), gentamicin (GEN), and imipenem (IMP) (all from the National Institutes for Food and Drug Control, Beijing, China). On the basis of the MICs, the S. flexneri isolates were classified as FOM-susceptible (MIC≤64 mg/L), FOM-intermediate (64 mg/L<MIC<256 mg/L), and FOMresistant (MIC≥256 mg/L). E. coli ATCC 25922 was used as the quality control strain. The susceptibility data of the Shigella isolates were accepted only if the MICs of the quality control strains tested in parallel were within the acceptable ranges set by the CLSI guidelines. Multi-drug resistance (MDR) was defined as resistance to three or more antimicrobial agents.
Conjugation experiments were performed for all isolates that were positive for fosA3, with sodium azide-resistant E. coli J53 as the recipient. Transconjugants were selected on MacConkey agar plates supplemented with sodium azide (200 mg/L) (Sigma Chemical Co., St. Louis, MO, USA), FOM (100 mg/L), and glucose-6-phosphate (25 mg/L). Transconjugants were tested by using biochemical methods and verified by using the API-20E system to be E. coli. Plasmid DNA was extracted from donors and transconjugants by using the Qiagen Plasmid Purification kit (QIAGEN, Hilden, Germany). The transconjugants were then screened by using PCR for fosA3 and β-lactamase genes, with the plasmid DNA as the template. The MICs of the following 11 antimicrobial agents were determined for the recipient, donors, and transconjugants: FOM, CTX, CRO, CAZ, CHL, PTZ, CIP, LEV, AMK, GEN, and IMP. All the assays were performed according to the protocols described in the previous section.
Incompatibility groups were assigned by using PCR-based replicon typing analysis of the transconjugants, according to the protocol described by Carattoli et al [13].
Of the 263 S. Flexneri isolates, 25 (9.5%) showed resistance to FOM, and 18 (6.8%) were positive for fosA3. fosC2 and fosA genes were not detected in any of the isolates. The antimicrobial resistance phenotypes of the fosA3-carrying isolates are shown in Table 1. All the isolates were resistant to FOM and CHL, while 16 of the 18 fosA3-carrying isolates (88.9%) exhibited MDR. The most prevalent antimicrobial resistance pattern was FOM-CHL-CTX-CRO. Seven (43.8%) and five (31.3%) of the MDR isolates were resistant to CIP and GEN, respectively, and all of them were susceptible to PTZ and IMP.
When screened for β-lactamase genes (Table 2), 17 (94.4%) of the fosA3-carrying isolates were CTX-M producers (CTX-M-55 [n=7], CTX-M-14 [n=5], and CTX-M-123 [n=5]), and three (16.7%) were TEM producers (TEM-1 [n=3]). In addition, the blaSHV gene was not detected in any of these isolates. Of the 18 fosA3-carrying isolates, three were found to be co-carrying blaCTX-M and blaTEM.
The plasmids from 13 of the 18 fosA3-carrying isolates (72.2%) were transferred into the recipient E. coli J53AzR by conjugation. The β-lactamase genes were co-transferred with the fosA3 gene to the recipient hrough plasmids. All the transconjugants co-carried fosA3 and blaCTX-M (CTX-M-55 [n=6], CTX-M-14 [n=4], and CTX-M-123 [n=3]). One plasmid from a transconjugant co-carried fosA3, blaCTX-M-55, and blaTEM-1. The MICs of the 11 antibiotics for the recipient, donors, and transconjugantsare presentedin Table 3. All the clinical isolates and transconjugants were resistant to FOM, and their resistance to CTX and CRO was greater than that to CAZ. The MICs of CTX for the transconjugants increased from <0.0625 µg/mL to around 16–32 µg/mL.
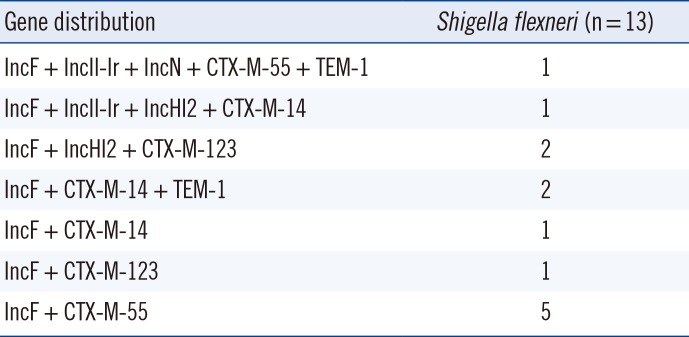
The replicon types of the fosA3-carrying plasmids in the 13 transconjugants were IncF (n=13, 100%), IncHI2 (n=3, 23.1%), IncIl-Ir (n=2, 15.4%), and IncN (n=1, 7.7%). Two transconjugants co-carried IncF and IncHI2, another one co-carried IncF, IncHI2, and IncIl-Ir, and another one co-carried IncF, IncN, and IncIl-Ir. The blaCTX-M-55 gene was carried by IncF (n=6), IncIl-Ir (n=1), and IncN (n=1) plasmids; the blaCTX-M-14 gene was carried by IncF (n=4), IncIl-Ir (n=1), and IncHI2 (n=1) plasmids; and the blaCTX-M-123 gene was carried by IncF (n=3) and IncHI2 (n=2) plasmids. The blaTEM-1 gene was associated with the IncF plasmid (Table 4).
To the best of our knowledge, this study is the first to characterize β-lactamase genes in fosA3-carrying S. flexneri isolates from patients in China. We showed that over 70% of FOM-resistant Shigella isolates harbored the fosA3 gene, which indicated that this gene was a part of the main mechanism responsible for FOM resistance. Our results also showed that 88.9% of fosA3-carrying Shigella isolates showed resistance to all commonly used antimicrobial agents. The most frequent antimicrobial resistance pattern was FOM-CHL-CTX-CRO. These findings confirmed that these antimicrobial agents were inefficient for the empirical treatment of patients infected by fosA3-carrying Shigella. The fosA3-carrying S. flexneri isolates were observed to be susceptible to PTZ and IMP, indicating that these antibiotics could be the preferred treatment options in Anhui, China.
This study also showed that 94.4% of the fosA3-carrying S. flexneri isolates collected from patients produced ESBLs, which was consistent with previous studies that showed that fosA3 was often found in CTX-M-producing E. coli isolates [678910]. The production of ESBLs is the major defense mechanism of the Shigella species against third-generation cephalosporins. Different types of β-lactamase genes, belonging to the TEM, SHV, and CTX-M families, have been reported in Shigella species worldwide [14151617]. In China, there have been several reports of β-lactamase genes in Shigella isolates, which produced CTX-M-14, CTX-M-15, CTX-M-55, and TEM-1 [14181920]. In the present study, CTX-M-55 was produced by 38.9% of the fosA3-carrying S. flexneri isolates, while CTX-M-14 was produced by 27.8%, CTX-M-123 by 27.8%, and TEM-1 by16.7% of the fosA3-carrying S. flexneri isolates. Thus, CTX-M-55 was the most prevalent ESBL produced by fosA3-carrying S. flexneri isolates in Anhui. CTX-M-123, which has been reported in E. coli isolates from animals in China, is a novel hybrid of the CTX-M-1 and CTX-M-9 group β-lactamases [21]. Notably, this is the first study to report CTX-M-123-type ESBLs in S. flexneri isolates from patients in China.
In this study, the fosA3 and blaCTX-M genes were co-carried by conjugative plasmids from multiple incompatible groups [678910]. We also showed that all the plasmids in the transconjugants co-carried fosA3 and blaCTX-M genes, and all transconjugants contained determinants encoding resistance to FOM, CTX, and CRO. The production of FosA3 and ESBLs, which were encoded on the conjugative plasmids, might explain the resistance to FOM and third-generation cephalosporins. Notably, one transconjugant co-carried fosA3, blaCTX-M-55, and blaTEM-1, and was resistant to FOM, CTX, CRO, and CAZ; it indicated that the plasmid-mediated fosA3 and ESBL genes played important roles in transferring resistance, and should be closely monitored.
Plasmid replicons associated with the fosA3 and blaCTX-M genes are known to vary with geographical location. For example, fosA3 and blaCTX-M genes have been reported to be harbored by IncF replicons in Korea and the United States [722], and by IncF, IncN, and IncI1 replicons in Japan and China [8911]. In the present study, the plasmid replicons of the13 transconjugants, which co-carried fosA3 and blaCTX-M, were IncF (n=13, 100%), IncHI2 (n=3, 23.1%), IncIl-Ir (n=2, 15.4%), and IncN (n=1, 7.7%); these results were consistent with the results for E. coli from most countries, suggesting that common mobilization events occurred in these bacteria.
In conclusion, this study showed that the prevalence of the plasmid-mediated fosA3 gene in S. flexneri isolates with FOM resistance was very high among patients in China. Moreover, the high prevalence of ESBL genes in fosA3-carrying isolates presents a serious threat to public health in Anhui, China. CTX-M-123 was reported in S. flexneri isolates from patients for the first time in China. Conjugatable plasmids were responsible for the dissemination of fosA3 and ESBL genes in S. flexneri isolates with high clonal diversity. Further studies will be needed to explore the mechanisms underlying the divergent evolution and the horizontal spread of plasmids that co-carried fosA3 and ESBL genes in MDR strains. To prevent potential outbreaks of MDR Shigella, the antimicrobial resistance in Shigella isolates should be continuously monitored, and rational antibiotic treatment, based on antimicrobial susceptibility tests, should be provided.
Acknowledgments
This work was supported by grants 81172737 and 81373072 from the National Natural Science Foundation of China. We would like to acknowledge the assistance of the 34 participating hospitals in collecting the isolates.
References
1. Christopher PR, David KV, John SM, Sankarapandian V. Antibiotic therapy for Shigella dysentery. Cochrane Database Syst Rev. 2010; CD006784.
2. Wang XY, Tao F, Xiao D, Lee H, Deen J, Gong J, et al. Trend and disease burden of bacillary dysentery in China (1991-2000). Bull World Health Organ. 2006; 84:561–568. PMID: 16878230.
3. Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010; 10:43–50. PMID: 20129148.
4. Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008; 46:1069–1077. PMID: 18444827.
5. Samonis G, Maraki S, Rafailidis PI, Kapaskelis A, Kastoris AC, Falagas ME. Antimicrobial susceptibility of Gram-negative nonurinary bacteria to fosfomycin and other antimicrobials. Future Microbiol. 2010; 5:961–970. PMID: 20521939.
6. Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother. 2010; 54:3061–3064. PMID: 20404116.
7. Lee SY, Park YJ, Yu JK, Jung S, Kim Y, Jeong SH, et al. Prevalence of acquired fosfomycin resistance among extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother. 2012; 67:2843–2847. PMID: 22893681.
8. Ho PL, Chan J, Lo WU, Lai EL, Cheung YY, Lau TC, et al. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J Med Microbiol. 2013; 62:1707–1713. PMID: 23988630.
9. Sato N, Kawamura K, Nakane K, Wachino J, Arakawa Y. First detection of fosfomycin resistance gene fosA3 in CTX-M-producing Escherichia coli isolates from healthy individuals in Japan. Microb Drug Resist. 2013; 19:477–482. PMID: 23909549.
10. Tseng SP, Wang SF, Kuo CY, Huang JW, Hung WC, Ke GM, et al. Characterization of fosfomycin resistant extended-spectrum β-lactamase-producing Escherichia coli isolates from human and pig in Taiwan. PLoS One. 2015; 10:e0135864. PMID: 26280832.
11. Hou J, Huang X, Deng Y, He L, Yang T, Zeng Z, et al. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M β-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob Agents Chemother. 2012; 56:2135–2138. PMID: 22232290.
12. Kim S, Kim J, Kang Y, Park Y, Lee B. Occurrence of extended-spectrum β-lactamases in members of the genus Shigella in the Republic of Korea. J Clin Microbiol. 2004; 42:5264–5269. PMID: 15528724.
13. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005; 63:219–228. PMID: 15935499.
14. Hu GZ, Chen HY, Si HB, Deng LX, Wei ZY, Yuan L, et al. Phenotypic and molecular characterization of TEM-116 extended-spectrum β-lactamase produced by a Shigella flexneri clinical isolate from chickens. FEMS Microbiol Lett. 2008; 279:162–166. PMID: 18093137.
15. Fortineau N, Naas T, Gaillot O, Nordmann P. SHV-type extended-spectrum β-lactamase in a Shigella flexneri clinical isolate. J Antimicrob Chemother. 2001; 47:685–688. PMID: 11328785.
16. Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005; 18:657–686. PMID: 16223952.
17. Taneja N, Mewara A, Kumar A, Verma G, Sharma M. Cephalosporin-resistant Shigella flexneri over 9 years (2001-09) in India. J Antimicrob Chemother. 2012; 67:1347–1353. PMID: 22410619.
18. Xiong Z, Li T, Xu Y, Li J. Detection of CTX-M-14 extended-spectrum β-lactamase in Shigella sonnei isolates from China. J Infect. 2007; 55:e125–e128. PMID: 17767959.
19. Xia S, Xu B, Huang L, Zhao JY, Ran L, Zhang J, et al. Prevalence and characterization of human Shigella infections in Henan Province, China, in 2006. J Clin Microbiol. 2011; 49:232–242. PMID: 21068291.
20. Zhang W, Luo Y, Li J, Lin L, Ma Y, Hu C, et al. Wide dissemination of multidrug-resistant Shigella isolates in China. J Antimicrob Chemother. 2011; 66:2527–2535. PMID: 21859815.
21. He D, Partridge SR, Shen J, Zeng Z, Liu L, Rao L, et al. CTX-M-123, a novel hybrid of the CTX-M-1 and CTX-M-9 Group β-lactamases recovered from Escherichia coli isolates in China. Antimicrob Agents Chemother. 2013; 57:4068–4071. PMID: 23752509.
22. Alrowais H, McElheny CL, Spychala CN, Sastry S, Guo Q, Butt AA, et al. Fosfomycin resistance in Escherichia coli, Pennsylvania, USA. Emerg Infect Dis. 2015; 21:2045–2047. PMID: 26488485.
Table 1
Antibiotic resistance patterns of 18 fosA3-carrying Shigella flexneri isolates from China
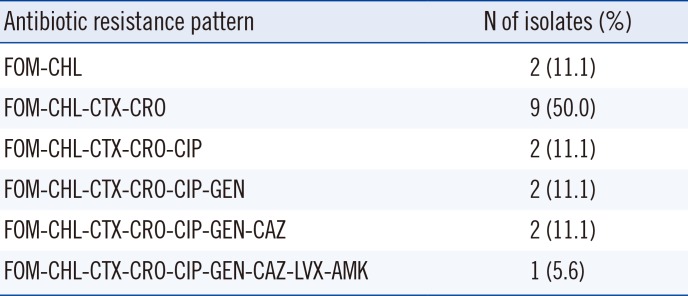
Table 2
Distribution of the genotypes of ESBLs in 17 fosA3-carrying Shigella flexneri isolates from China
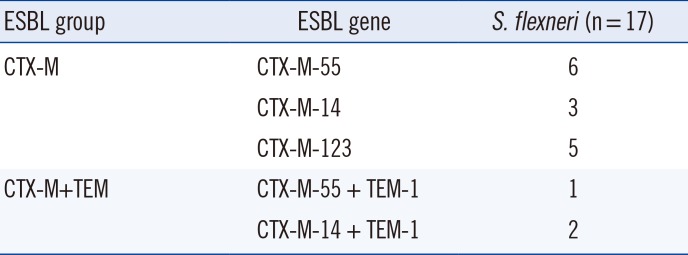
| ESBL group | ESBL gene | S. flexneri (n = 17) |
|---|---|---|
| CTX-M | CTX-M-55 | 6 |
| CTX-M-14 | 3 | |
| CTX-M-123 | 5 | |
| CTX-M+TEM | CTX-M-55 + TEM-1 | 1 |
| CTX-M-14 + TEM-1 | 2 |
Table 3
MIC50 and MIC90 of 11 antimicrobial agents for Shigella flexneri isolates and transconjugants
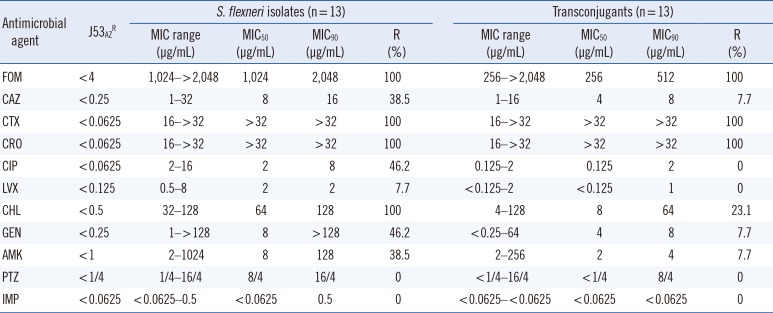
Abbreviations: FOM, fosfomycin; CHL, chloramphenicol; CTX, cefotaxime; CRO,ceftriaxone; CIP, ciprofloxacin; GEN, gentamicin; CAZ, ceftazidime; LVX, levofloxacin; AMK, amikacin; PTZ, piperacillin-tazobactam; IMP, imipenem; J53AzR, sodiumazide-resistant E. coli J53; MIC, minimal inhibitory concentration; R, resistance.
Table 4
Distribution of the genotypes of replicons and β-lactamase genes in 13 fosA3-carrying transconjugants




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download