This article has been
cited by other articles in ScienceCentral.
Abstract
The College of American Pathologists (CAP) offers a suite of laboratory accreditation programs, including one specific to accreditation to the international organization for standardization (ISO) 15189 standard for quality management specific to medical laboratories. CAP leaders offer an overview of ISO 15189 including its components, internal audits, occurrence management, document control, and risk management. The authors provide a comparison of its own ISO 15189 program, CAP 15189, to the CAP Laboratory Accreditation Program. The authors conclude with why laboratories should use ISO 15189.
Keywords: ISO 15189, Quality management, Laboratory accreditation, College of American Pathologists
INTRODUCTION
Drawing on the collective knowledge of more than 650 experts in laboratory medicine, the College of American Pathologists (CAP) helps laboratories navigate the accelerating changes in laboratory medicine and health care through our integrated laboratory improvement programs. These programs focus on accreditation and proficiency testing (PT) to ensure the highest quality of patient care and mitigate risk of noncompliance where applicable. The CAP is proud of its accreditation programs, with the core CAP Laboratory Accreditation Program (LAP) launched more half a century ago. Today, the CAP accredits more than 8,000 laboratories in all of its accreditation programs, including approximately 437 international laboratories in more than 50 countries. Other CAP accreditation programs include Forensic Drug Testing, Reproductive, Biorepository, and CAP 15189. CAP 15189 is a quality management program the CAP designed for accreditation to the international organization for standardization (ISO) 15189 standard, building on the longstanding CAP LAP program. As such, the laboratory must first be accredited in the CAP LAP before seeking accreditation to the ISO 15189 standard with CAP 15189. Accreditation to the ISO 15189 standard does not meet US Clinical Laboratory Improvement Amendments (CLIA) requirements and cannot replace a CLIA-based accreditation for laboratories in the United States. The CAP offers the following overview of ISO 15189 from the chair of the CAP 15189 Committee, Frank Schneider, MD, FCAP, a pathologist at the Kaiser Foundation Hospital in Oakland, California, and Caroline Maurer, MT (ASCP), PMP, director of the CAP 15189 Program in Northfield, Illinois. The overview is adapted from the original chapter in Schneider F., Maurer C. (2017). ISO 15189 In Qihui Z., Siegal G.P. (Eds.),
Quality Management in Anatomic Pathology:
Strategies for Assessment,
Improvement,
and Assurance (pp. 185-193). Northfield, IL: College of American Pathologists [
1].
ISO 15189: An Overview from the College of American Pathologists
ISO 15189 standard, Medical laboratories - Requirements for quality and competence, hereafter referred to as ISO 15189, was first published in 2003 and revised in 2007 and again in 2012. ISO 15189 is not a tool merely to meet accreditation requirements or provide quick fixes for individual mistakes. Instead, laboratories implementing ISO 15189 strive to:
Create systems that are as failure resistant as possible, will catch mistakes before they become a problem, and reduce errors by getting things right the first time
Identify opportunities for improvement at all times
Involve and empower their staff by involving them in the solving of problems and the implementation of solutions
ISO 15189 encourages full involvement and utilization of the abilities of all employees at all levels to improve the organization. In a laboratory accredited to ISO 15189, the goal is continual improvement, and for staff members to know exactly what to do, how to do it, who is in charge of a process, and where to find all information necessary to perform their jobs.
By 2015, about 60 countries had made ISO 15189 part of their mandatory medical laboratory accreditation requirements. In the United States, accreditation to ISO 15189 is voluntary, as no governmental or regulatory agency requires laboratories or health care providers to conform to ISO 15189.
Components of the ISO 15189 quality management system
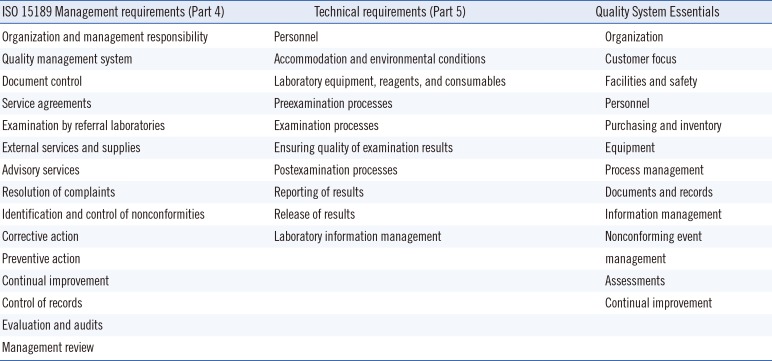
The ISO 15189 requirements, or clauses, fill merely 31 pages (plus a few pages of definitions and appendices). ISO 15189 is divided into management requirements (Part 4, focusing on the quality management system [QMS] structure, function, and effective management of laboratory operations, its quality system, guiding policies, and processes) and technical requirements (Part 5, focusing on the technical competency and related procedures and processes).
Table 1 summarizes the content of the ISO 15189 standard, Parts 4 and Part 5 [
23].
ISO 15189 is a comprehensive standard, offering an overarching structure to laboratory operations. It applies to all divisions of a medical laboratory, regardless of the services it provides or the way it is organized; the standard is as relevant in a full-service medical laboratory as it is in a laboratory providing services exclusively for either clinical or anatomic pathology. It is not prescriptive in the sense that it does not specify how to address a particular requirement or clause. Its focus and importance lies in encouraging users to maintain an effective quality management system integrated across all parts of their operation, with a goal of continual improvement.
The management requirements demand regular management reviews and internal audits to assure that the laboratory's activities adhere to the QMS, are effective, and continually meet clients' needs, and to identify opportunities for improvement before issues arise. Laboratory management has to take a step back and view its operation holistically, including appropriateness, suitability, and performance of testing; relations to suppliers, clinicians, patients, and consultants; and detecting nonconformities, finding their causes, and implementing effective solutions.
The technical requirements reflect many elements from the CAP LAP accreditation checklists, but in a more generic format. The focus of the standard here, just as in the management section, is implementation and effectiveness. To those trying to implement ISO 15189 in their laboratory, it will soon become clear that nonconformance to technical requirements can often be traced back to nonconformance in the laboratory's QMS and potentially highlights broader process issues than the incidental transaction.
It would be counter to the idea of ISO 15189 to consider some components of the standard more important than others. For the purpose of this article, we chose to highlight the following four elements of ISO 15189 because we find that their effective implementation is often the most novel and challenging, while also the most rewarding aspect of ISO 15189.
Internal audits
ISO 15189 is a systems- and process-oriented QMS. It encourages systematic identification of processes and their interrelatedness. Proper documentation of core processes helps reveal the structure of the operation. The understanding of the interactions of processes enhances effectiveness and efficiency because it uncovers gaps and unnecessary activities. Assessing implementation and effectiveness of process is a major, if not the most important, element of a thorough ISO 15189 accreditation assessment.
ISO 15189-accredited laboratories have to perform internal audits of their QMS on a regular basis. Such audits need to include the managerial and technical components, as well as preexamination, examination, and postexamination processes. These serve to:
Ascertain that all activities of the QMS are covered (“Are we adhering to our own quality system?”)
Assure that processes are effective (“Does every consultation report requested by a pathologist always end up being reported in an addendum?”)
Identify opportunities for improvement (“Can we establish criteria with our oncologists when to perform reflex molecular testing rather than having to wait for a request to initiate testing?”)
ISO 15189-accredited laboratories usually perform internal audits by section, with an entire cycle completed within one year. Increased frequency of audits may be required depending on risk and occurrence management outcomes.
Internal audit results are one of several inputs for the regular, high-level management review required of ISO 15189-accredited laboratories. Management review meetings offer the opportunity to review issues that impact the laboratory's processes, including, but not limited to, internal audit results, quality metrics, ongoing quality improvement projects, opportunities for improvement, complaints, and forthcoming new technologies or regulatory changes.
Quality management decisions in an ISO 15189 system are based on facts and data. This prevents personal preferences or a top-down management style from inhibiting process and quality improvement.
Continual improvement is a permanent objective of ISO 15189 quality management. This does not mean, for example in anatomic pathology that every conceivable effort should be made to reduce the frozen section turnaround time by 1 min. The laboratory should find meaningful metrics that are aligned with the laboratory's mission. Improvements may affect any aspect of the quality management system and its processes. They may involve issues such as saving control tissue for immunohistochemical stains, making the work instructions for lymphoma workups more succinct and accessible, or automating frozen section versus final diagnosis correlations using the laboratory information system.
Similarly, in clinical chemistry, continual improvement does not mean making every conceivable effort to reduce hemoglobin turnaround time by 10 sec. However, it may mean making efforts to drive down variation and risk, which can help a core laboratory avoid repeating testing, incorrect results, physician complaints, or even possibly having to add a satellite lab with its associated overhead costs.
More importantly, continual improvement also refers to the improvement of the quality management system, for example (1) performing better, more thorough internal audits; (2) increasing the use of personal protective equipment (and thus better implementation of safety procedures) in the gross room to prevent eye splashes or scalpel injuries; or (3) sending staff members to training courses for performing root cause analysis, an integral part of the standard for occurrence management.
Occurrence management
Occurrence management refers to the resolution of nonconformities. Nonconformities are routinely identified through a plethora of sources, the most important of which include internal and external audits as well as errors made. The central theme of occurrence management in an ISO 15189 QMS is root cause analysis (RCA) [
4]. RCA is often thought of as one step in the investigation of a major, potentially life-threatening, patient safety event. RCA in the context of ISO 15189 has a very different connotation. In an ISO 15189-accredited laboratory, RCA is a commonly and regularly used tool addressing a wide variety of problems, ranging from the most minor (usually recurring) to the most complex (usually rare). ISO 15189-accredited laboratories view RCA as an opportunity to brainstorm, think of solutions “outside the box,” and make improvements. In general, the scope and magnitude of any corrective action should be appropriate to the nonconformity and its effects.
RCA also provides an opportunity to engage staff in the process of quality improvement. Staff is viewed as a most valuable asset and should always be considered part of the solution, rather than part of a problem. As a corollary, errors in an ISO 15189-accredited laboratory are not attributed to individuals, but to process failures. By definition, removing the root cause(s) of a nonconformity should prevent its recurrence. Blaming and retraining staff following an error usually does not eliminate the risk of recurrence in the long term. In an ISO 15189 QMS, an RCA is a component of corrective action that serves to redesign and “mistake-proof” a process, implement those process changes, and assure through effectiveness checks that the changes actually solved the problem. The universal and liberal use of RCA is a strong point of ISO 15189.
Document control
Most laboratories are struggling with document control, evidenced by its being a common deficiency cited in CAP LAP inspections. Work aids (or “cheat sheets”), if necessary, must be included under document control and reference the complete procedure. They also become subject of internal and external audits. Document control is more than having procedures signed on time, eliminating cheat sheets with outdated information, and finding a document during an accreditation visit. Document control means that pathologists and other staff have the correct information needed for a task readily available when needed. This reduces risk of error, need to rework, incorrect results, time spent on fixing mistakes, and cost. For example, a laboratory scientist receiving an unexpected flow cytometry sample on a Saturday afternoon should be able to find the information on how to submit the material in the correct medium with the right form to the right section in the shortest amount of time required for successful acquisition.
Risk management
ISO 15189 states, “The laboratory shall evaluate the impact of work processes and potential failures on examination results as they affect patient safety, and shall modify processes to reduce or eliminate the identified risks and document decisions and actions taken” [
1].
Insights into existing or potential risks come from several sources.
When a new process is developed, the laboratory will identify the process owner, map the process, and identify its risk points.
In most cases, risks will be identified during internal or external audits (eg, inspections or PT events).
Review of the occurrence management data will allow identification of future risks based on past errors.
If the environment and culture of the organization support it, staff at all levels will feel comfortable and find it worthwhile to bring up potential risks identified during routine events and everyday observation (eg, near-misses that may otherwise get no follow-up, or unofficial “water-cooler talk” about an upcoming change in another department that may affect the laboratory).
No matter how risks are identified, in an ISO 15189 QMS, each risk will be assessed on the basis of its probability, severity, and impact on patient care. The laboratory should then discuss and agree upon the level of risk it is willing to accept. In the vast majority of instances, risk cannot be eliminated. The ISO 15189-accredited laboratory will take appropriate steps to control risks. This may require mistake-proofing of an existing process or complete redesign of a process if risk is severe. Care must be taken that process changes do not introduce new, unanticipated risks.
Relationship of CLIA and ISO 15189 accreditation
Accreditation to the ISO 15189 standard does not meet US CLIA requirements and cannot replace a CLIA-based accreditation. ISO 15189 could be chosen as the QMS of a CLIA laboratory, but it needs to be supplemented with the specific CLIA requirements. Because elements of CLIA were incorporated into the ISO 15189 standard, many laboratories considering implementing ISO 15189 can readily meet many of the technical requirements. We find that the management requirements are often more difficult to implement, but we cannot stress enough that their implementation, driving effective outcomes and improved patient care, are the most rewarding aspect of an ISO 15189 QMS.
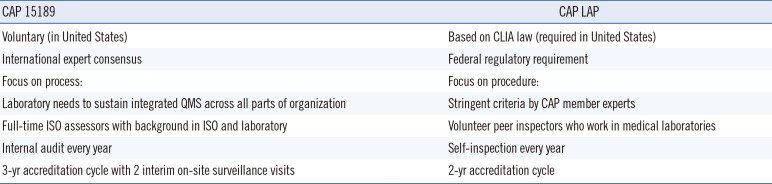
Table 2 compares features of the CAP 15189 program with the CAP LAP.
A laboratory must first be accredited in the CAP LAP before seeking accreditation to the ISO 15189 standard with the CAP. When a laboratory applies to the CAP 15189 accreditation program, the assessment team first conducts an off-site review of the laboratory's documents in order to evaluate the components of the QMS and the laboratory's readiness for accreditation. The laboratory also performs an internal audit in order to assess readiness on its own.
Prior to the on-site accreditation assessment, the laboratory has the option to undergo a gap assessment. A gap assessment is a detailed on-site assessment against the ISO 15189 standard and adherence to the laboratory's QMS that reveals holes in the system and issues that need to be addressed before accreditation could be granted.
During the actual on-site accreditation assessment, the CAP examines in detail the QMS and its implementation. Nonconformities have to be addressed before accreditation can be granted, requiring an RCA, a plan on how to fix the problem, and a plan to check whether the fix was effective. A committee, comprised mostly of practicing pathologists, renders an accreditation decision after review of the laboratory's corrective actions. If accreditation is granted, the laboratory is ISO 15189 accredited for three years.
Justifying ISO 15189 with return on investment
The value of implementing and sustaining an ISO 15189 QMS is not easy to quantify. The literature is sparse. Firstly, variability in the medical laboratory industry is high. Secondly, it would be difficult to show statistically significant differences in outcome measures because of this complexity. Furthermore, what constitutes appropriate outcome measures is controversial in itself. Over the last nine years in which we have been involved with laboratories that implemented ISO 15189 quality management systems, we have found the following elements to be of value to laboratories.
Engaging in ISO 15189 is a journey that requires many years of commitment. We often hear that “things” become easier the longer the laboratory pursues this course. The culture of the laboratory changes gradually to enable a mindset of “seeing” problems as, or before, they occur, therefore evolving to a culture of prevention. Staff engagement improves and morale improves, as there is no blame and staff members are part of the solution. Reductions in LAP inspection deficiencies have been demonstrated as a result of investing in ISO 15189 [
5].
Financial reward is a desirable and most-often-sought argument in favor of ISO 15189. Although the literature of money-savings in an ISO 15189 system is sparse, cost-of-quality models have demonstrated potential cost savings [
678]. Such models consider the cost of trying to be the best you can be (cost of pathologists' continuing medical education or money spent on process design), the cost of maintaining a quality operation (eg, accreditation program expenses or enrollment of pathologists in PT programs), the cost of correcting process failures before the result is reported (eg, cost of histology having to recut skin biopsies to obtain a full face), and the cost of resolving problems after reporting results (eg, cost of pathologist's time to amend a report because operating room staff indicated the wrong site on a requisition form). Using ISO 15189 methods to reduce the cost allows laboratories to estimate expected cost savings and financial benefits to gain high-level management support and stakeholder buy-in.
ISO 15189 requires the right attitude and mindset -- those who practice it like to think of it as a “lifestyle choice,” rather than just another accreditation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download