Abstract
Background
The associations of vitamin D deficiency with various clinical conditions highlighted the importance of vitamin D testing. Currently, clinicians measure only the total 25-hydroxyvitamin D [25(OH)D] concentration, regardless of its bioavailability. We aimed to determine the effect of vitamin D-binding protein (VDBP) on 25(OH)D bioavailability.
Methods
Serum samples were collected from 60 healthy controls, 50 pregnant women, and 50 patients in intensive care units (ICUs). Total 25(OH)D was quantified by liquid chromatography with tandem mass spectrometry, and VDBP levels were determined by using an ELISA kit (R&D Systems, USA). The bioavailable 25(OH)D levels were calculated by using total 25(OH)D, VDBP, and albumin concentrations.
Results
In comparison with healthy controls, the total 25(OH)D concentration was significantly lower in ICU patients (median, 11.65 vs 18.25 ng/mL; P<0.00001), but no significant difference was noted between pregnant women (18.25 ng/mL) and healthy controls. The VDBP level was significantly lower in ICU patients (95.58 vs 167.18 µg/mL, P=0.0002) and higher in pregnant women (225.01 vs 167.18 µg/mL, P=0.008) compared with healthy controls. Nonetheless, the calculated bioavailable 25(OH)D levels of ICU patients and pregnant women were significantly lower than those of healthy controls (1.97 and 1.93 ng/mL vs 2.56 ng/mL; P=0.0073 and 0.0027).
Conclusions
A single marker of the total 25(OH)D level is not sufficient to accurately evaluate vitamin D status, especially in pregnant women. In cases where VDBP concentrations may be altered, VDBP measurements and bioavailable 25(OH)D calculations may help to determine vitamin D status accurately.
Humans can acquire vitamin D from exposure to sunlight and dietary intake. Acquired vitamin D is metabolized in the liver, resulting in 25-hydroxyvitamin D [25(OH)D] [12]. Most (85-90%) of the circulating 25(OH)D is tightly bound to vitamin D-binding protein (VDBP), with a smaller amount (10-15%) loosely bound to albumin. Less than 1% of circulating vitamin D exists in a free unbound form [345]. The fraction not bound to VDBP (free and albumin-bound forms) is considered bioavailable 25(OH)D [3].
VDBP, a 58-kDa protein, is produced in the liver and circulates in plasma. VDBP levels increase by up to 50% in a high-estrogen state, such as pregnancy, and decrease in certain disease states, such as severe hepatic disease [6789]. Moreover, VDBP has a high rate of polymorphism, and differences in affinity for 25(OH)D have been reported [10].
Vitamin D deficiency is defined as a serum 25(OH)D concentration less than 20 ng/mL (50 nmol/L) [211]. Several studies have shown that vitamin D deficiency correlates with various clinical conditions and parameters, including osteoporosis, cardiovascular diseases, autoimmune diseases, schizophrenia, surgical outcomes, and cancer risk [1213141516171819]. Nonetheless, the causality is still unproven. Because vitamin D bioavailability is linked to various conditions, accurate assessment of vitamin D status has become important for effective treatment.
Currently, most clinicians determine total 25(OH)D levels as a measure of the reservoir of vitamin D. However, the exact bioavailability of vitamin D is not known by this method. Our aim was to test whether VDBP quantification and calculation of bioavailable vitamin D are helpful in the assessment of vitamin D status in patients likely to have altered VDBP levels, including pregnant women and patients in intensive care units (ICUs).
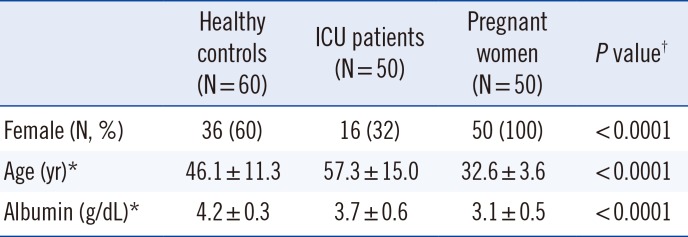
This was a prospective study conducted in Konkuk University Hospital, Seoul, Korea. Serum samples were collected from 160 patients between March and September 2014. The 160 enrolled patients were categorized as healthy controls (n=60), ICU patients (n=50), or pregnant women (n=50) [8]. The healthy controls were defined as people who visited a health care institution only for the health screenings and did not have any symptoms. All the ICU patients included in this study had been admitted for postoperative care only. Pregnant women who had no medical problems were recruited, and those with twins or triplets were excluded. Shao et al [20] discovered the significant difference in parathyroid hormone and calcium levels between early and late stages of pregnancy. To evaluate the differences by pregnancy stage, the group of pregnant women was subdivided into two subgroups; 1st and 2nd/3rd trimesters. In all three groups, patients who had other medical problems or took medications were excluded. Table 1 shows the demographics of the three study groups. This study was approved by the Institutional Review Board of the Konkuk University Medical Center (KUH1200040).
Each serum sample was aliquoted into two tubes and stored at -80℃ until they could be analyzed for VDBP and total 25(OH)D. Concentrations of VDBP were measured by using Human Vitamin D BP Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. The 25(OH)D2 and 25(OH)D3 concentrations were measured by using liquid chromatography with tandem mass spectrometry (LC-MS/MS). LC-MS/MS was regarded as a reference method and was performed at a College of American Pathologists-accredited laboratory. The D2 and D3 values were added up to determine total 25(OH)D. The coefficients of variation of D2 and D3 measured by LC-MS/MS were as follows; D2: 4.2% for a low level, 3.8% for a high level; D3: 4.0% for a low level, 3.5% for a high level. Serum albumin levels were measured by using an enzymatic colorimetric method with a TBA-200FR (Toshiba, Tokyo, Japan). The levels of bioavailable 25(OH)D were calculated from total measured 25(OH)D, VDBP, and serum albumin concentrations by using the following equations [48]:
Data were expressed as median [interquartile range] or mean± standard deviation. Total 25(OH)D, VDBP, and calculated bioavailable 25(OH)D were compared among healthy controls, ICU patients, and pregnant women by using the Kruskal-Wallis test with post hoc pairwise comparisons. To compare total 25(OH)D, VDBP, and calculated bioavailable 25(OH)D by gestational stage, the Mann-Whitney test was used. For categorical variables, the Fisher's exact test was used. Statistical analysis was performed by using the Analyse-it software, version 3.90.5 (Analyse-it Software Ltd., Leeds, UK) and MedCalc software, version 15.2.2 (MedCalc Software bvba, Ostend, Belgium). Differences with P values less than 0.05 were considered statistically significant.
ICU patients had total 25(OH)D levels (median, interquartile range: 11.65, 7.86-14.87 ng/mL; SI unit, nmol/L; conversion factor, 2.496) that were significantly lower than those of healthy controls (18.25, 13.48-23.78 ng/mL; P<0.0001) and pregnant women (18.25, 13.98-25.24 ng/mL; P<0.0001; Fig. 1A). The VDBP level of pregnant women (225.01, 130.24-422.92 µg/mL) was significantly higher than that of healthy controls (167.18, 105.99-257.70 µg/mL, P=0.008), while the VDBP level of ICU patients (95.58, 61.15-167.34 µg/mL, P=0.0002) was significantly lower than that of healthy controls (Fig. 1B). Finally, the calculated bioavailable 25(OH)D levels in ICU patients (1.97, 1.48-3.15 ng/mL, P=0.0073) and pregnant women (1.93, 1.03-3.41 ng/mL, P=0.0027) were significantly lower than those in healthy controls (2.56, 1.95-4.22 ng/mL; Fig. 1C).
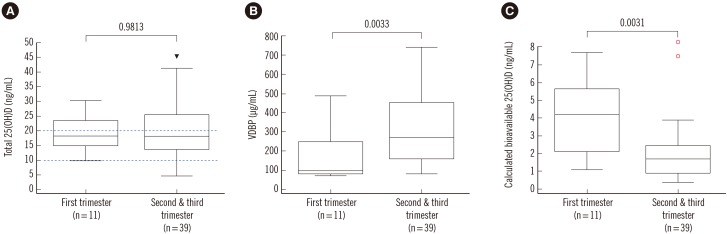
In pregnant women, the total 25(OH)D levels were not significantly different between the 1st trimester and the 2nd and 3rd trimesters (1st trimester: 18.20, 15.07-23.87 ng/mL, 2nd and 3rd trimesters: 18.30, 13.75-25.70 ng/mL; Fig. 2A). In contrast, VDBP levels during the 2nd and 3rd trimesters were significantly higher than those in the 1st trimester (1st trimester: 102.20, 84.75-259.90 µg/mL, 2nd and 3rd trimesters: 273.65, 163.75-453.98 µg/mL, P=0.0033; Fig. 2B). Thus, the calculated bioavailable 25(OH)D level in the 2nd and 3rd trimesters of pregnancy was significantly lower than that in the 1st trimester (1st trimester: 4.19, 2.09-5.67 ng/mL, 2nd and 3rd trimesters: 1.73, 0.90-2.43 ng/mL, P=0.0031; Fig. 2C).
Correlations between vitamin D deficiency and a variety of clinical conditions have been reported in numerous studies [1112131415161718]. Vitamin D deficiency is defined as total 25(OH)D concentration less than 20 ng/mL, regardless of its bioavailability. Because VDBP levels can be altered by changes in estrogen levels or by hepatic diseases, the actual vitamin D status may have a different relation with the total 25(OH)D concentration in patients with abnormal VDBP level [6792122]. However, there are no studies on the calculated bioavailable 25(OH)D levels in pregnant women and ICU patients with changed VDBP level. In this study, we evaluated the clinical utility of calculated bioavailable 25(OH)D from VDBP measurements for estimation of vitamin D status in ICU patients without hepatic diseases, in pregnant women, and in healthy controls.
The mean total 25(OH)D concentration of the healthy control group was 19.26 ng/mL, and 36 of 60 individuals in this group (60%) received a diagnosis of vitamin D deficiency. This result suggests that vitamin D deficiency is common among city dwellers, even in the presumably healthy population without apparent diseases. The mean VDBP level of the group of ICU patients was significantly lower than that of the healthy control group. This result suggests that VDBP production may be reduced during postoperative care, even in the absence of hepatic disease.
Heijboer et al [8] discovered decreased VDBP levels in ICU patients, compared with healthy individuals. In the present study, ICU patients also showed median total 25(OH)D and VDBP levels that are significantly lower than those in the control group individuals. Depending on the degree of VDBP downregulation, bioavailable 25(OH)D concentrations may increase or decrease, theoretically. As a result, total 25(OH)D is not sufficient as a single marker of vitamin D status.
The pregnant group showed a median total 25(OH)D level comparable to that of the healthy control group. Nonetheless, the calculated bioavailable 25(OH)D level in pregnant women was significantly lower than that in healthy controls (Fig. 1). These results suggest that vitamin D deficiency may be overlooked in pregnant women, if only the total 25(OH)D level is measured. Moreover, the possibility of misdiagnosis would be higher during the 2nd and 3rd trimester, as the bioavailable 25(OH)D decreases (Fig. 2).
There are some limitations in this study. First, the difference in affinity of VDBP for 25(OH)D, which results from genetic variations, was not taken into account in our calculation of 25(OH)D bioavailability [8922]. Second, patients with hepatic diseases were not included. Third, the reference range for the calculated bioavailable 25(OH)D was not established. Fourth, there were statistically significant differences in age, gender, and albumin levels among our groups (Table 1). Because young age and female sex are associated with high VDBP levels [2324] and the bioavailable 25(OH)D calculation includes the albumin level, our results on the differences in VDBP levels and in the calculated bioavailable 25(OH)D levels among the groups could be affected by these differences in characteristics of the study subjects. Nonetheless, the calculated bioavailable 25(OH)D may reflect the status of VDBP levels and the albumin levels in each patient; therefore, calculated bioavailable 25(OH)D could be used to assess vitamin D status accurately in the patients with altered VDBP levels or albumin levels. Fifth, the concentration of VDBP can be measured only by an ELISA kit, making commercial application potentially challenging. Sixth, we could not measure the levels of calcium, parathyroid hormone, and creatinine because of sample volume limitations. It should be noted that Shao et al [20] found significantly higher parathyroid hormone and lower calcium levels at the late pregnancy stage (32.01±3.45 weeks) in comparison with the early pregnancy stage (16.09±2.65 weeks). This result seems to be consistent with the relatively prominent vitamin D deficiency in women at the late stage of pregnancy. Seventh, we could not verify the VDBP concentration by a second method. Because the median value of VDBP concentration (167.18 µg/mL) in the healthy controls (composed of Asian people in our study) was found to be similar to that in American Blacks (mean±SE: 168±3 µg/mL) in another study [22], and allele frequencies of the VDBP (GC) polymorphism in keratinized (yellowish) skin type are closer to those in pigmented (black) than those in fair-skinned populations [25], we assumed that there were no major errors in VDBP measurement. Because quantification of VDBP and free 25(OH)D is widely available to practitioners in current clinical practice, further studies and the assays/calculations improvements should be performed to address the above limitations.
In conclusion, a single marker of total 25(OH)D levels has long been used to evaluate vitamin D status in patients. This measure, however, is not sufficient to accurately evaluate a patient's vitamin D status, especially that of pregnant women. In cohorts of patients with altered VDBP concentrations, such as pregnant women or ICU patients, VDBP testing and bioavailable 25(OH)D calculations would provide a more accurate assessment of vitamin D status.
References
1. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004; 80(6 Suppl):S1689–S1696.
2. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281. PMID: 17634462.
3. Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012; 82:84–89. PMID: 22398410.
4. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986; 63:954–959. PMID: 3745408.
5. Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985; 61:969–975. PMID: 3840175.
6. Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012; 30:445–456. PMID: 22528806.
7. Bhan I. Vitamin D binding protein and bone health. Int J Endocrinol. 2014; 2014:561214. PMID: 24987416.
8. Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012; 58:543–548. PMID: 22247500.
9. Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, et al. A comparison of meassured and calculated and free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014; 99:1631–1637. PMID: 24483159.
10. Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet. 1993; 92:183–188. PMID: 8370586.
11. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011; 86:50–60. PMID: 21193656.
12. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heavey RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007; 85:1586–1591. PMID: 17556697.
13. Mitchell D. The relationship between vitamin D and cancer. Clin J Oncol Nurs. 2011; 15:557–560. PMID: 21951742.
14. Mann MC, Hollenberg MD, Hanley DA, Ahmed SB. Vitamin D, the autonomic nervous system, and cardiovascular risk. Physiol Rep. 2015; 3:e12349. PMID: 25902783.
15. Garlanad CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006; 96:252–261. PMID: 16380576.
16. Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002; 181-182:71–78. PMID: 12505287.
17. McGrath J, Selten JP, Chant D. Long-term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration - data from Australia and the Netherlands. Schizophr Res. 2002; 54:199–212. PMID: 11950544.
18. Iglar PJ, Hogan KJ. Vitamin D status and surgical outcomes: a systematic review. Patient Saf Surg. 2015; 9:14–23. PMID: 25926889.
19. Cleal JK, Day PE, Simner CL, Barton SJ, Mahon PA, Inskip HM, et al. Placental amino acid transport may be regulated by maternal vitamin D and vitamin D-binding protein: results from the Southampton Women's Survey. Br J Nutr. 2015; 113:1903–1910. PMID: 25940599.
20. Shao H, Tao M, Fan Y, Jing J, Lu J. Vitamin D levels and other factors related to bone mineral density during pregnancy. Aust N Z J Obstet Gynaecol. 2012; 52:571–575. PMID: 23003672.
21. Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin-D binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981; 67:589–596. PMID: 6894152.
22. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013; 369:1991–2000. PMID: 24256378.
23. Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxy vitamin D levels in serum from normal subject, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984; 74:1966–1971. PMID: 6549014.
24. Pop LC, Shapses SA, Chang B, Sun W, Wang X. Vitamin D-binding protein in healthy pre- and postmenopausal women: relationship with estradiol concentrations. Endocr Pract. 2015; 21:936–942. PMID: 26121448.
25. Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986; 72:281–293. PMID: 3516862.
Fig. 1
Comparison of total 25-hydroxyvitamin D [25(OH)D], vitamin D binding protein (VDBP), and calculated bioavailable 25(OH)D in the three study groups. (A) The total 25(OH)D level in intensive care unit (ICU) patients (median, interquartile range: 11.65, 7.86-14.87 ng/mL) was significantly lower than that in healthy controls (18.25, 13.48-23.78 ng/mL) or in pregnant women (18.25, 13.98-25.24 ng/mL). (B) The VDBP level in pregnant women (225.01, 130.24-422.92 µg/mL) was significantly higher, and the VDBP level in ICU patients (95.58, 61.15-167.34 µg/mL) was significantly lower than that in healthy controls (167.18, 105.99-257.70 µg/mL). (C) The calculated bioavailable 25(OH)D levels of ICU patients (1.97, 1.48-3.15 ng/mL) and pregnant women (1.93, 1.03-3.41 ng/mL) were significantly lower than those in healthy controls (2.56, 1.95-4.22 ng/mL). P values were calculated by the Mann-Whitney test. Two dashed lines denote vitamin D deficiency and severe vitamin D deficiency. The arrowheads and dots represent the outside value (>1.5× interquartile ranges) and far-out value (>3× interquartile ranges), respectively. The horizontal lines represent maximum and minimum values, except for the outside value and far-out value.
Abbreviation: ICU, intensive care unit.
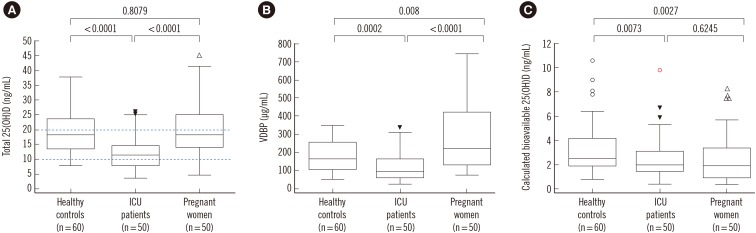
Fig. 2
Comparison of total 25-hydroxyvitamin D [25(OH)D], vitamin D-binding protein (VDBP), and calculated bioavailable 25(OH)D by gestational stage. (A) Total 25(OH)D levels were not significantly different between the 1st and 2nd and 3rd trimesters (median, interquartile range: 1st trimester: 18.20, 15.07-23.87 ng/mL; 2nd and 3rd trimesters: 18.30, 13.75-25.70 ng/mL). (B) VDBP levels during the 2nd and 3rd trimesters were significantly higher than those during the 1st trimester (1st trimester: 102.20, 84.75-259.90 µg/mL, 2nd and 3rd trimesters: 273.65, 163.75-453.98 µg/mL). (C) The calculated bioavailable 25(OH)D level in the 2nd and 3rd trimesters was significantly lower than that in the 1st trimester (1st trimester: 4.19, 2.09-5.67 ng/mL; 2nd and 3rd trimesters, 1.73, 0.90-2.43 ng/mL). P values were calculated by the Mann-Whitney test. Two dashed lines denote vitamin D deficiency and severe vitamin D deficiency. The arrowheads and dots represent the outside value (>1.5× interquartile ranges) and far-out value (>3× interquartile ranges), respectively. The horizontal lines represent the maximum and minimum values, except for the outside value and far-out value.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


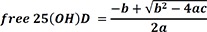
 XML Download
XML Download