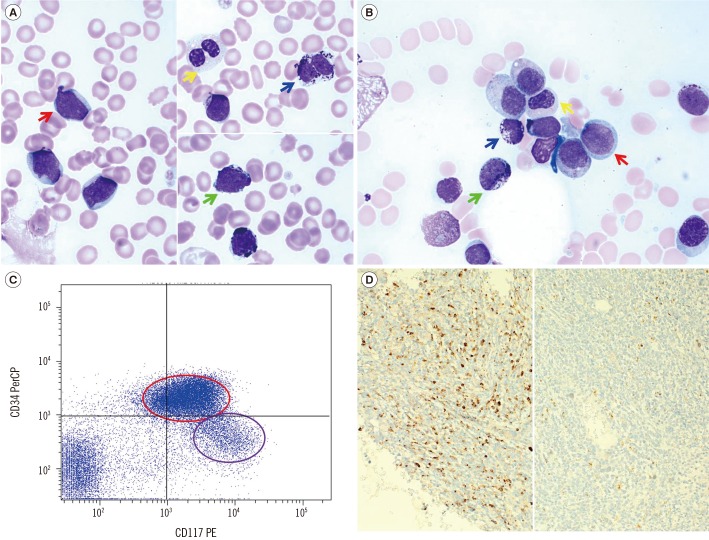
 | Fig. 1Morphology and phenotype of neoplastic cells. (A) Blood smears revealed non-mast cell blasts (red arrow), a dysplastic neutrophil with the pelgeroid change (yellow arrow), an atypical mast cell exhibiting bi-lobed nuclei (blue arrow), and heavily granulated blast cells suggesting metachromatic blasts (light green arrow; Wright-Giemsa stain, ×1,000). (B) Bone marrow aspirate smears revealed a blast that was medium-sized with round nuclei, finely dispersed chromatin, some distinct nucleoli, and a small amount of basophilic cytoplasm without granules (red arrow). Bone marrow aspirate smears also demonstrated mast cells, including granulated blasts (light green arrow) and atypical mast cells with bi-lobed nuclei (blue arrow). Dysplastic features such as decreased granules were seen in the granulocytic lineage (yellow arrow; Wright-Giemsa stain, ×1,000). (C) The population of neoplastic cells in the blast gate separated into two subgroups, i.e., myeloblasts with CD34+/CD117+ (red circle) and immature atypical mast cells with CD34−/CD117+ (purple circle). (D) Histologic examination of a bone marrow trephine specimen showed a diffuse interstitial increase in mast cells instead of formation of dense clusters (left half; immunohistochemistry for tryptase, ×200). There is no evidence of CD25 positivity (right half; immunohistochemistry for CD25, ×200).Abbreviations: PerCP, peridinin-chlorophyll proteins; PE, phycoerythrin.
|
Dear Editor,
AML is occasionally associated with bone marrow (BM) mastocytosis. Differential diagnoses include reactive mast cell (MC) hyperplasia, systemic mastocytosis (SM) with associated clonal hematological non-MC lineage disease, and MC leukemia (MCL) [1]. Myelomastocytic leukemia (MML) is a new classification for AML patients who show an increase in immature and atypical MCs, but who do not fulfill the criteria for MCL or SM [234567]. Here, we describe for the first time a case of therapy-related MML, and review key elements of differential diagnosis in patients with myeloid neoplasms and BM mastocytosis [89].
A 59-yr-old woman presented with fever. She had a history of ovarian carcinoma, which was treated by total abdominal hysterectomy, mass excision, and subsequent administration of the paclitaxel/taxol and carboplatin/carbo (TC) regimen 10 yr prior to the presentation. Five years later, she developed peritoneal metastasis and received additional chemotherapy, including gemcitabine and carboplatin (GEMC), repeated TC, peglylated liposomal doxorubicin (PLD), and bevacizumab. Overt tumor spread was repressed during chemotherapy, and the patient remained in stable condition. However, abdominal computed tomography (CT) scan performed at the current visit revealed recurrent peritoneal seeding of carcinoma. The patient had white blood cell counts of 33.7×109/L, hemoglobin level of 8.7 g/dL, and platelet counts of 43×109/L. The peripheral blood smear identified 28% blasts and 40% immature MCs. The BM aspirate smear showed dysplastic features with 46.0% myeloblasts and 22.0% immature atypical MCs (Fig. 1A, 1B). Flow cytometry analysis showed that the blasts were CD34+, HLA-DR+, CD13+, CD33+, CD117+, CD65+, CD15+, CD7dim, CD2−, CD3−, cCD3−, cCD22−, CD10−, CD19−, and TdT−. A small population of immature atypical MCs was also identified, which was phenotypically similar to the blast population, but was CD34−, CD65−, and CD7− (Fig. 1C). BM core biopsy sections were packed with immature cells and had a diffuse MC infiltrate. The immature cells were negative for CD25 (Fig. 1D). Cytogenetic analysis revealed a complex karyotype: 44,XX,−5,−7,inv(9)(p12q13),del(12)(p13),add(18)(p11.3)[11]/43,idem,−X,−3,+4[14]. No mutations were detected in FLT3, NPM1, CEBPA, or KIT. Serum tryptase level was not tested. The latency period from first exposure to carboplatin, an alkylating agent, was approximately 10 yr, and the duration of chemotherapy by this time was 33 months. She was diagnosed as having therapy-related AML according to the 2008 WHO classification [10] and MML according to a recent consensus proposal [89]. The patient declined further chemotherapy or surgical intervention and was transferred to another hospital for supportive care.
Although the overall incidence of MML is unknown, it is clear that the disease is very rare [9]. The first diagnostic criterion of MML is an increase in MC along with myeloblasts in the blood and BM. In this context, morphologically defined subsets of MCs, as described in the consensus proposal and summarized below, can be useful for differential diagnosis: 1) typical mature tissue MCs; 2) atypical type I spindle-shaped MCs; 3) atypical type II MCs, also referred to as promastocytes; and 4) metachromatic blasts [8]. MML is characterized by an increase in the latter two subsets. Histological findings in MML include diffuse spread of MCs, as opposed to SM where MCs form multifocal aggregates. Furthermore, MCs in MML usually lack CD25 and CD2, whereas those in SM aberrantly express CD25 and/or CD2. These findings suggest that the immunohistochemical panel for detecting AML with mastocytosis should at least include CD117, tryptase, CD25 and/or CD2. The presence of KIT D816V mutation excludes the diagnosis of MML [89].
There are several reasons underscoring the importance of distinguishing MML from other leukemia subtypes with BM mastocytosis. First, it has been suggested that MCs in MML are derived from a malignant clone. A previous study showed that both the tryptase-negative blasts cells and the MCs were found to contain the RUNX1-RUNX1T1 fusion gene [3]. Second, this disease may be mistaken for several other diseases. Without refinement of the diagnostic criteria for MML, hematopathologists might have difficulties in distinguishing between different differential diagnoses in patients with mastocytosis. Third, MML is associated with poor prognosis [9]. This results partially from a complex karyotype and myelodysplasia, as has been reported here as well as in previous patients with MML [7]. Identification of patients at high risk of developing MML can be the starting point for personalized therapy.
In conclusion, although MML poses a diagnostic challenge and is not yet listed in the current WHO classification, a meticulous evaluation of blood and BM smears and a thorough immunohistochemical study of BM trephine specimens facilitate the MML diagnosis.
Notes
Authors' Disclosures of Potential Conflicts of Interest: No conflicts of interest relevant to this article were reported
Go to : 
References
1. Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, et al. Mastocytosis. In : Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2008. p. 54–63.
2. Valent P, Sperr WR, Samorapoompichit P, Geissler K, Lechner K, Horny HP, et al. Myelomastocytic overlap syndromes: biology, criteria, and relationship to mastocytosis. Leuk Res. 2001; 25:595–602. PMID: 11377685.
3. Sperr WR, Drach J, Hauswirth AW, Ackermann J, Mitterbauer M, Mitterbauer G, et al. Myelomastocytic leukemia: evidence for the origin of mast cells from the leukemic clone and eradication by allogeneic stem cell transplantation. Clin Cancer Res. 2005; 11:6787–6792. PMID: 16203765.
4. Arredondo AR, Gotlib J, Shier L, Medeiros B, Wong K, Cherry A, et al. Myelomastocytic leukemia versus mast cell leukemia versus systemic mastocytosis associated with acute myeloid leukemia: a diagnostic challenge. Am J Hematol. 2010; 85:600–606. PMID: 20658589.
5. Intzes S, Wiersma S, Meyerson HJ. Myelomastocytic leukemia with t(8;21) in a 3-year-old child. J Pediatr Hematol Oncol. 2011; 33:e372–e375. PMID: 22042288.
6. Johnson RC, Savage NM, Chiang T, Gotlib JR, Cherry AM, Arber DA, et al. Hidden mastocytosis in acute myeloid leukemia with t(8;21)(q22;q22). Am J Clin Pathol. 2013; 140:525–535. PMID: 24045550.
7. Rich A, Sun J, Aldayel AS, Yin CC, Medeiros LJ, Konoplev S. Myelomastocytic leukemia with aberrant CD25 expression: case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2014; 14:e173–e177. PMID: 25022599.
8. Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014; 25:1691–1700. PMID: 24675021.
9. Horny HP, Sotlar K, Reiter A, Valent P. Myelomastocytic leukemia: histopathological features, diagnostic criteria and differential diagnosis. Expert Rev Hematol. 2014; 7:431–437. PMID: 25025369.
10. Vardiman JW, Arber DA, Brunning RD, Larson RA, Matutes E, Baumann I, et al. Therapy-related myeloid neoplasms. In : Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2008. p. 127–129.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download