Abstract
Cytomegalovirus (CMV) is a well-established cause of morbidity and mortality in pediatric recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT). CD8+ T-cells are important for controlling CMV infection. We conducted a prospective pilot study to investigate the clinical utility of measuring the CMV-specific T-cell immune response using the QuantiFERON-CMV assay (QF-CMV) in pediatric allo-HSCT recipients. Overall, 16 of 25 (64%) patients developed CMV infection. QF-CMV was evaluated in these 16 patients during the early and late phases of the first CMV infection post allo-HSCT. Whereas the initial QF-CMV results during the early phase of CMV infection did not correlate with the course of the corresponding infection, the QF-CMV results post resolution of the first CMV infection correlated with the recurrence of CMV infection until 12 months post allo-HSCT; no recurrent infections occurred in the four QF-CMV-positive patients, while recurrent infections manifested in five of eight QF-CMV-negative (62.5%) and all three QF-CMV-indeterminate patients (P=0.019). In spite of the small number of patients examined, this study supports the potential application of monitoring CMV-specific T-cell immunity using the QF-CMV assay to predict the recurrence of CMV infection in pediatric allo-HSCT recipients.
Cytomegalovirus (CMV) is a well-known cause of morbidity and mortality in pediatric allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients [123]. CMV presents with a variety of clinical manifestations in allo-HSCT recipients, from asymptomatic infection to CMV diseases such as interstitial pneumonia, gastroenteritis, hepatitis, retinitis, and encephalitis [1]. Prophylactic and preemptive antiviral therapy has been used to prevent and control CMV infection [4]. However, prolonged antiviral therapy following allo-HSCT is associated with various side effects, development of drug-resistant strains, and late onset CMV disease [567].
Cell-mediated immunity is known to play a major role in controlling CMV infection [8]. Although both CD4+ and CD8+ T-cells are important for controlling and restricting viral infection, CD8+ T-cells are thought to be the most important component of the immune control of CMV via the production of interferon-gamma (IFN-γ) [89]. Therefore, measuring the IFN-γ secreted by CMV-specific T-cells may be useful for identifying patients who may benefit from prophylactic antiviral therapy [10].
The QuantiFERON-CMV assay (QF-CMV) (Qiagen, Chadstone, VIC, Australia) detects IFN-γ released from T cells, particularly CD8+ T cells stimulated with CMV peptides, by ELISA; this assay can be used for in vitro quantification of cell-mediated immune responses to CMV peptide antigens associated with immune control of CMV infection [11]. Previous studies have shown this method to be useful for assessing the risk of late-onset CMV disease in solid organ transplant recipients and for predicting the occurrence of CMV infection in allo-HSCT recipients [1213141516]. However, data in pediatric patients are relatively limited [17]. The present pilot study aimed to investigate the clinical utility of measuring CMV-specific T-cell immunity response by QF-CMV in pediatric allo-HSCT recipients.
Thirty-three pediatric patients who underwent allo-HSCT between April 2011 and March 2013 were enrolled. Written informed consent was obtained from all patients and/or their parents. Eight patients were excluded for refusing consecutive blood sample collection during CMV infection episodes. Finally, 25 patients were included in this study and their clinical course was followed longitudinally for 12 months post allo-HSCT. The six patients who died during the follow-up period were not excluded because mortality was not due to CMV infection. Routine virological surveillance of CMV was conducted by using CMV antigenemia assay twice or thrice a week until six months post HSCT, and then once a week until 12 months. QF-CMV was performed at four weeks post allo-HSCT for baseline data. If patients became CMV antigenemia reactive, indicating the presence of CMV infection, QF-CMV was measured simultaneously with CMV antigenemia and continued until the end of the antiviral treatment, seven days subsequent to CMV antigenemia becoming negative. This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea.
CMV serostatus was assessed in both donors and recipients prior to transplantation by detecting the presence of IgG and/or IgM antibodies using the VIDAS CMV IgG and IgM kit (bioMerieux, Hazelwood, MO, USA). CMV-infected peripheral blood leukocytes were detected by using the CMV pp65 antigenemia direct fluorescence assay with the CINA Kit system (Argene Biosoft, Varilhes, France) and a monoclonal antibody against the CMV pp65 antigen. CMV infection was defined as the presence of more than one pp65-positive cell per 200,000 leukocytes (white blood cells [WBCs]) on a slide. CMV infection duration was defined as the period from the date that CMV antigenemia was reported as ≥1 cell/200,000 WBCs to the first of three consecutive time points at which CMV antigenemia was reported as 0 cells/200,000 WBCs. QF-CMV was performed according to the manufacturer's instructions. Briefly, whole blood was collected into three QF-CMV blood collection tubes: Nil Control, CMV Antigen, and Mitogen. The tubes were incubated for 16 to 24 hr at 37℃. Following incubation, plasma was obtained by centrifugation and the amount of IFN-γ (IU/mL) in the plasma was measured by using ELISA. The QF-CMV results were interpreted by using the following criteria: Positive, CMV minus Nil ≥0.2 IU/mL; Negative, CMV minus Nil <0.2 IU/mL and Mitogen minus Nil ≥0.5 IU/mL; Indeterminate, CMV minus Nil <0.2 IU/mL and Mitogen minus Nil <0.5 IU/mL. The differences between the three result groups were analyzed by using Fisher's exact test for categorical variables and Kruskal-Wallis test for continuous variables. Statistical analysis was performed by using IBM SPSS version 23.0 (IBM, Armonk, NY, USA), and P values <0.05 were considered statistically significant.
Sixteen of the 25 patients (64.0%) experienced CMV infection during the first year post allo-HSCT. Eight of these 16 patients had recurrent infection episodes; two patients had two episodes, five patients had three episodes, and one patient had four episodes. The median time to the first infection was 22 days post-HSCT (range, 11 to 53 days), the median duration was 19 days (range, 5 to 47 days), and the median maximum CMV antigenemia was 17 cells/200,000 WBCs (range, 1 to 720 cells/200,000 WBCs). The clinical and demographic characteristics of the 25 patients are summarized in Supplemental Data Table S1. No differences were observed between the parameters of patients with and without CMV infection (including donor and recipient CMV serostatus and recipient four week post-allo-HSCT QF-CMV), except HLA match status (P=0.01).
The initial QF-CMV of the 16 CMV infected patients, measured during the early phase of the first CMV infection, was positive in four, negative in four, and indeterminate in eight patients. The maximum CMV antigenemia levels and the duration of the corresponding infection were not statistically significant (P=0.421 and P=0.740, respectively) in the three result groups (Table 1). However, the QF-CMV results measured following the resolution of the first CMV infection were significantly associated with the subsequent recurrence of CMV infection. No recurrent CMV infection was observed in the four QF-CMV-positive patients, while five of the eight QF-CMV-negative (62.5%) and three of the three QF-CMV-indeterminate (100.0%) patients experienced recurrent CMV infection (P=0.019) (Table 2). The test results of one patient were not available.
Three representative patient cases are shown in Fig. 1 to delineate the longitudinal patterns of CMV-specific T-cell immunity in relation to CMV infection, defined by the presence of CMV antigenemia. The first two patients, who exhibited indeterminate and/or negative results in early phases of infection but were repeatedly QF-CMV-positive at the end of the CMV infection, did not experience recurrent infection thereafter until 12 months post allo-HSCT (Fig. 1A, B). However, the third patient who had never converted to positive and exhibited persistent QF-CMV-negative results, except for a single positive result during the early transplant phase, had recurrent infection episodes (Fig. 1C).
In this pilot study, we examined the clinical utility of the CMV-specific T-cell immunity test using QF-CMV to predict recurrent CMV infections in pediatric allo-HSCT recipients within the first year post-transplantation. Patients with positive QF-CMV results following initial CMV infection had no recurrent infections thereafter, whereas a significant proportion of patients with indeterminate or negative results had recurrent CMV infections. These findings indicate that the established CMV-specific T-cell immunity following initial CMV infection contributes to the prevention of recurrent CMV infection episodes. In addition, we assessed the ability of QF-CMV to control CMV infection using the maximum CMV antigenemia level and duration of CMV infection as a measure of CMV infection control. A previous study including adult allo-HSCT recipients demonstrated that patients with positive QF-CMV measured immediately following CMV infection had a lower median maximum CMV load compared with patients exhibiting negative QF-CMV [12]. However, we did not observe a significant difference in control of CMV infection, in terms of maximum CMV antigenemia level and duration of CMV infection among the three QF-CMV result groups. This might be due to the small number of patients in our study or differences in patient demographics, diagnoses, and treatment.
The proportion of indeterminate results (50.0%) measured during the early phase of CMV infection is higher than in the total collected samples (37.5%); this is similar to previously reported results (33% [15] and 38% [12]) and is probably due to lymphopenia during the early phase post HSCT. Three of the eight patients with indeterminate results during CMV infection showed very high CMV antigenemia levels (maximum 101, 277 and 720 cells/200,000 cells, respectively), which emphasizes the importance of reconstituting general immunity to acquire pathogen specific immunity.
QF-CMV would be successfully incorporated into the clinical setting; it is a simpler test than other assays, such as ELISPOT, MHC-peptide tetramer staining, and intracellular cytokine assay, for the detection of IFN-γ secreted in response to CMV-specific CD8+ T-cell activation. Although this pilot study had some limitations, such as a small number of patients and heterogeneous diagnoses, to the best of our knowledge, this is the first report to examine Korean pediatric patients who underwent allo-HSCT with systematic longitudinal monitoring scheme for 12 months post allo-HSCT. The results of this study strongly support the potential clinical application of monitoring CMV-specific T-cell immunity using the QF-CMV assay to predict the recurrence of CMV infection in pediatric allo-HSCT recipients. Further studies with larger number of patients are required to establish the practical applications of QF-CMV in the pediatric allo-HSCT setting.
References
1. Hebart H, Einsele H. Clinical aspects of CMV infection after stem cell transplantation. Hum Immunol. 2004; 65:432–436. PMID: 15172442.
2. Castagnola E, Cappelli B, Erba D, Rabagliati A, Lanino E, Dini G. Cytomegalovirus infection after bone marrow transplantation in children. Hum Immunol. 2004; 65:416–422. PMID: 15172440.
3. Ljungman P. CMV infections after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008; 42(Sl):S70–S72. PMID: 18724309.
4. Meijer E, Boland GJ, Verdonck LF. Prevention of cytomegalovirus disease in recipients of allogeneic stem cell transplants. Clin Microbiol Rev. 2003; 16:647–657. PMID: 14557291.
5. Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993; 118:173–178. PMID: 8380242.
6. Goodrich JM, Boeckh M, Bowden R. Strategies for the prevention of cytomegalovirus disease after marrow transplantation. Clin Infect Dis. 1994; 19:287–298. PMID: 7986901.
7. Boeckh M, Fries B, Nichols WG. Recent advances in the prevention of CMV infection and disease after hematopoietic stem cell transplantation. Pediatr Transplant. 2004; 8(S5):S19–S27.
8. Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009; 22:76–98. PMID: 19136435.
9. Harari A, Zimmerli SC, Pantaleo G. Cytomegalovirus (CMV)-specific cellular immune responses. Hum Immunol. 2004; 65:500–506. PMID: 15172450.
10. Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant. 2007; 40:865–869. PMID: 17724444.
11. Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis. 2007; 9:165–170. PMID: 17462006.
12. Tey SK, Kennedy GA, Cromer D, Davenport MP, Walker S, Jones LI, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One. 2013; 8:e74744. PMID: 24146744.
13. Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013; 56:817–824. PMID: 23196955.
14. Lochmanova A, Lochman I, Tomaskova H, Marsalkova P, Raszka J, Mrazek J, et al. Quantiferon-CMV test in prediction of cytomegalovirus infection after kidney transplantation. Transplant Proc. 2010; 42:3574–3577. PMID: 21094818.
15. Fleming T, Dunne J, Crowley B. Ex vivo monitoring of human cytomegalovirus-specific CD8(+) T-Cell responses using the QuantiFERON-CMV assay in allogeneic hematopoietic stem cell transplant recipients attending an Irish hospital. J Med Virol. 2010; 82:433–440. PMID: 20087937.
16. Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009; 9:1214–1222. PMID: 19422346.
17. Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, et al. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation. 2012; 93:536–542. PMID: 22314338.
Supplementary Material
Supplemental Data Table S1
Characteristics of the patients according to the presence or absence of CMV infection after allogeneic HSCT
Fig. 1
The longitudinal patterns of CMV pp65 antigenemia and CMV-specific T-cell response measured by using the QuantiFERON-CMV assay in three allogeneic HSCT recipients. (A) Nine-year-old female patient with aplastic anemia with repeated positive QuantiFERON-CMV assay results during the early phase of CMV infection. (B) Eleven-month-old male patient with juvenile myelomonocytic leukemia who converted from indeterminate and negative to positive QuantiFERON-CMV results during the course of infection. (C) Twenty-eight-month-old female patient with neuroblastoma with persistent negative results, except for a single positive result during the early transplant phase; the patient died because of tumor progression at 140 days post HSCT. ‘+’ indicates a positive QF-CMV result, ‘-’ indicates a negative result, and ‘♦’ indicates an indeterminate result.
Abbreviations: CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation; WBCs, white blood cells.
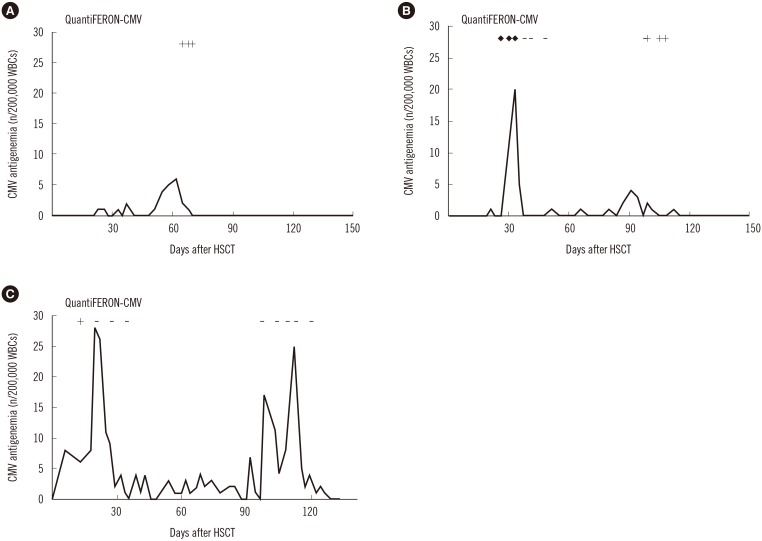
Table 1
Maximum CMV antigenemia and duration of CMV infection in patients with positive, negative, and indeterminate initial QuantiFERON-CMV results measured during the early phase of the first CMV infection post allogeneic HSCT
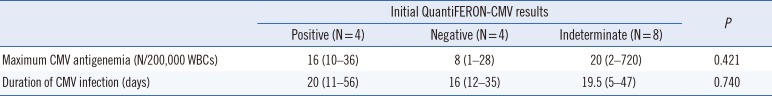
Table 2
Occurrence of recurrent CMV infection in patients with positive, negative, and indeterminate QuantiFERON-CMV results measured at the end of the first CMV infection post allogeneic HSCT





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download