Abstract
Background
Grafts survive despite blood group antigens on the transplant being continuously exposed to antibodies in the blood of recipients in ABO-incompatible kidney transplantation (ABOi KT), owing to the mechanism of accommodation. We analyzed the immunodynamics of soluble ABH antigens in allografts from secretor donors and the influence of such immunodynamics on accommodation and subsequent graft survival in ABOi KT.
Methods
The genotype of a known human β-galactoside α-1,2-fucosyltransferase gene (FUT2), which determines soluble ABH antigen secretor status, was established in 32 donors for ABOi KT at the Severance Hospital, from June 2010 to July 2015. Clinical outcomes of recipients, such as anti-A/B antibody titer change, renal function, and graft survival, were evaluated.
Results
Twenty-five donors were secretors (78.1%), and seven were nonsecretors (21.9%). The frequency of anti-A/B IgG or IgM antibody titer elevation or reduction post-transplantation was not significantly related to donor secretor status. However, IgM titer was rapidly reduced in recipients transplanted from nonsecretor donors (P=0.01), which could be explained by the lack of absorption effect of soluble antigens, enhancing the binding of antibodies to antigens in the allografts. Interestingly, soluble ABH antigens did not affect rejection-free graft survival, which may be due to the nature of β-galactoside α-1,2-fucosyltransferase.
ABO-incompatible kidney transplantation (ABOi KT), a method first introduced in the 1970s and widely adopted in the 1990s, has expanded the donor pool by increasing the availability of transplantable organs and has decreased the waiting period for a kidney [1]. Initially, the success of ABOi KT was hindered by naturally occurring anti-A/B (anti-A and/or anti-B) antibodies that had not been removed from circulation at the time of transplantation; these caused hyperacute rejection in preliminary cases [2]. However, the development of potent immunosuppressive agents and multiple plasma exchanges allows anti-A/B antibody titers to be reduced prior to transplantation [3], leading to a decrease in the rate of acute rejection; from the mid-1980s to today, the rate of acute rejection has decreased to less than 10% during the first year following transplantation [45].
During ABOi KT, the pre-existing anti-A/B antibodies target the ABH antigens of the transplanted kidney, which are expressed on the vascular endothelium, distal convoluted tubules, and collecting tubules [6]. The expression of ABH antigens in human tissues differs according to ABO blood group and secretor status. Water-soluble ABH antigens are expressed solely in the epithelial cells of the collecting tubules and the calyceal epithelium of secretors [7]. In addition, kidney glycolipid antigens have higher expression in secretor-positive individuals [8]. The human β-galactoside α-1,2-fucosyltransferase gene (FUT2) encodes the β-galactoside α-1,2-fucosyltransferase function necessary for the synthesis of ABH antigens in the kidney [910].
Previous studies have suggested that early graft loss after ABOi-KT may be due to attack of recipient-specific anti-A/B antibodies, including ABH antigens [711]. This phenomenon was later referred to as antibody-mediated rejection (ABMR) and defined as allograft rejection caused by antibodies directed against donor-specific antigens [12]. The term ‘accommodation’ was introduced in an attempt to explain why some kidney grafts fail while others survive incompatible transplantation [13]. Accommodation refers to the phenomenon in which an antigen-antibody reaction does not occur, despite the presence of antigens on the graft and the existence of antibodies in the blood of the recipient [14]. The most compelling mechanism explaining accommodation is ‘protection’ against the recipients' antibodies, which involves changes that occur in the graft in response to injury caused by antibody binding and complement activation [15]. On the basis of several suggested protection mechanisms, we decided to investigate the effects of neutralization by ABH substances released from the donor kidney in relation to secretor status and the influence of this effect on recipient accommodation. To the best of our knowledge, this study is the first to examine the relation between immunodynamics during accommodation and donor secretor status using genotyping during ABOi-KT.
Of the 126 patients who had undergone living donor ABOi KT at Severance Hospital, Seoul, Korea from June 2010 to July 2015, 32 adult recipients with available donor blood samples and negative pretransplant lymphocyte cross-match tests were enrolled in this retrospective study. The study was approved by the Institutional Review Board (IRB) of Severance Hospital (approval no. 4-2015-0201) for the retrospective chart reviews and studies of existing preserved specimens. The demographics of subjects according to the donors' secretor status were assessed by reviewing hospital records and laboratory results (Table 1).
Donor FUT2 (NM_000511.5) genotypes were determined by Sanger sequencing using FUT2-specific oligo primers that could detect the c.357C>T, c.385A>T, c.428G>A, and fusion gene polymorphisms. The functional alleles of FUT2 are Se and Se357, whereas se385, se357,385, se428, and sefus have been reported as non-functional alleles [16]. We designed the oligonucleotide primers to amplify 600 bp product for FUT2 as follows: 5′-CAGCGGCTAGCGAAGATTCAA-3′ (sense) and 5′-CCAGTCCAGGGCCTGCTGTA-3′ (antisense).
All patients underwent a pretransplantation conditioning protocol consisting of therapeutic plasma exchange (TPE) followed by administration of intravenous immunoglobulin (IVIG) (100 mg/kg) and immunosuppressants (0.1 mg/day tacrolimus, 1,500 mg/day mycophenolate, 20 mg/day prednisone, and 375 mg/m2 rituximab). All patients received pretransplantation conditioning prior to the operation. TPE was administered to 27 patients with an anti-A/B antibody titer greater than 1:8 using the COBE spectra system (Terumo BCT, Lakewood, CO, USA). One plasma volume was removed from each patient, and 100% replacement was provided by using a 5% albumin solution or AB blood group fresh frozen plasma. TPE was performed by using 5% albumin solution for the initial sessions, and the last two sessions of TPE were carried out with the AB blood group fresh frozen plasma (FFP) to prevent bleeding before transplantation. TPE and IVIG treatments were conducted every other day before transplantation until both IgM and IgG titers were no greater than 1:8. Immunosuppressive drugs were used before transplantation to prevent graft rejection. Administration of tacrolimus, mycophenolate, and prednisone was initiated seven days before transplantation, and rituximab was administered two days before transplantation after performing TPE [3].
Anti-A/B antibody titers were determined by the tube method, in which two-fold serial dilutions of the patients' serum were tested with 3% Affirmagen A/B indicator red cells for IgG and IgM (Ortho Diagnostics, Rochester, NY, USA) [17]. After incubation at room temperature for 30 min and centrifugation at 973g for 15 sec, the highest serum dilution ratio that showed 1+ reactivity indicated the anti-A/B antibody titers. IgG titers were measured by using serum samples treated with 0.01M dithiothreitol solution (Sigma-Aldrich, St. Louis, MO, USA), while IgM titers were determined from untreated samples and read by a single technician at the same facility to ensure accuracy. Antibody titers were evaluated daily following initiation of the conditioning protocol while preparing for transplantation, and postoperation titers were determined regularly in patients [3]. One patient, whose antibody titers were not evaluated post-transplantation, was excluded from the titer outcome analysis.
Serum creatinine levels were measured regularly to estimate graft function by using the Jaffe method on a Hitachi 7600-210 autoanalyzer (Hitachi Co. Ltd., Tokyo, Japan) using Sekisui's Creatinine reagent (Sekisui Diagnostics, Stamford, CT, USA).
The initial anti-A/B antibody titer was defined as the recipient anti-A/B antibody titer prior to any immunomodulatory conditioning, such as TPE, IVIG, and immunosuppressant therapy. The baseline anti-A/B titer antibody was defined as the recipient anti-A/B antibody titer immediately prior to transplantation. One patient exhibiting reduction following postoperative TPE was excluded from the analysis because the titer could have been affected by the treatment.
In order to investigate the immunodynamics of soluble ABH antigens in allografts from secretor donors that result in accommodation, we focused on small fluctuation in anti-A/B antibody titers. Titer elevation was defined as one or more Δlog2 titer elevation after transplant from the baseline Δlog2 titer at transplantation and the time period (days) between at least one Δlog2 titer elevation after transplant from the baseline log2 titer at transplantation was counted. Similarly, titer reduction was defined as one or more Δlog2 titer reduction after transplant from the baseline log2 titer at transplantation, and the time period (days) between at least one Δlog2 titer reduction after transplant from the baseline log2 titer at transplantation was counted.
Patients with clinically suspected acute rejection exhibiting either greater than 20% increase in serum creatinine levels compared with baseline levels or clinical symptoms (e.g., oliguria or edema), underwent a biopsy. Rejection or other pathological findings were diagnosed according to the Banff 2011 criteria [18].
The graft rejection-free survival rate was investigated at two time points; that at one month represented the accommodation period, and that at one year represented general graft maintenance.
Statistical analyses were performed by using SPSS software (version 23.0; SPSS Inc., Chicago, IL, USA). Medians of non-normally distributed quantitative values were compared by Mann-Whitney U tests, and Pearson's chi-square tests and Fisher's exact tests were used to compare nominal variables. Graft rejection-free survival rates among patients' group according to donors' secretor status were compared using Kaplan-Meier analysis, by log-rank testing. A two-tailed P value of less than 0.05 was considered statistically significant.
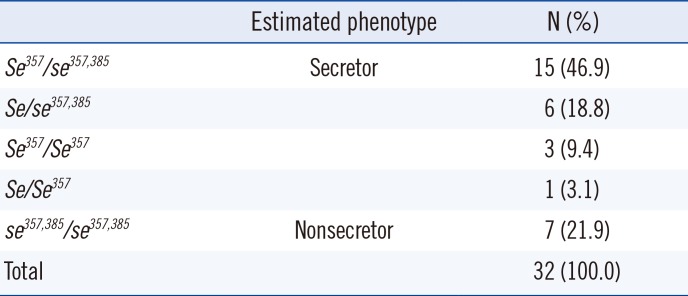
The secretor statuses of the donors are summarized in Table 2. Individuals carrying the four common genotypes, i.e., Se357/se357,385, se357,385/se357,385, Se/se357,385, and Se357/Se357, accounted for 97% of the study population. Allele frequencies of the donor secretor genotypes, i.e., Se, Se357, se357,385, se428, and sefus, were 0.016, 0.438, 0.547, 0.000, and 0.000, respectively.
As shown in Table 3, of the 31 patients, 24 (77.4%) and 22 (71.0%) patients exhibited one or more elevations or reductions in anti-A/B IgG and IgM antibody titers post-transplantation, respectively, compared with the baseline following preconditioning. Reductions in anti-A/B IgG and IgM antibody titers were only observed in four (12.9%) and 10 (32.3%) patients post-transplant, respectively. However, the frequencies of titer elevations and reductions were not significantly different.
The time (days) required for a one-fold elevation in IgG and IgM or reduction in IgG was not significantly different among groups; however, the time (days) required for a one-fold reduction in IgM was significantly different, with IgM titers reduced significantly earlier in recipients of nonsecretor donors (RNSD)(P=0.01; Fig. 1).
All recipients exhibited reduced serum creatinine levels post-transplantation, usually within the first week. Prior to transplantation, serum creatinine levels were 2.98–15.77 mg/dL in all patients. Following transplantation, serum creatinine dropped 84.6%±6.9% in the recipients of secretor donors (RSD) and 83.4%±7.6% in the RNSD group (P=0.713). Minimum creatinine levels were observed at 4.9±1.8 days in the RSD group and at 3.0±0.0 days in the RNSD group (P=0.154; Table 3).
Biopsies were performed in 13 patients who were clinically suspected of having rejection, including 11 patients in the RSD group (44.0%) and two patients in the RNSD group (28.6%). Of the patients with biopsy-proven rejection, a mixed form of ABMR and T-cell-mediated rejection (TCMR) was observed in two patients who received kidneys from RSD, while isolated ABMR and TCMR were noted in two and three patients, respectively. Interstitial fibrosis and tubular atrophy were observed in only five recipients who had received kidneys from RSD. However, in the case of ABMR, the severity was different in two patients; one patient in the RNSD group was stage III at day 12 (g1 t0 i1 v3 cg0 ct0 ci0 cv0 mm0 ah0 ptc2), while the other patient in the RSD group was stage I at day 9 (g0 t0 i0 v0 cg0 ct1 ci0 cv0 mm0 ah0 ptc0).
Fig. 2A and B shows differences in graft rejection-free survival within one month and one year post-transplantation between the RSD and RNSD groups. The differences in survival rates of recipients in the RSD and RNSD groups were not significant at both time points (95% confidence interval, 27.8–31.6, P=0.960 for one month; 286.4–359.8, P=0.943 for one year).
The kidney, one of the most vascularized organs, is not immunologically privileged in transplantation because of its endothelium, which expresses donor allo-antigens, and because it is readily accessible to circulating antibodies [19]. However, once the formidable ABO-incompatibility barrier is overcome by B-cell depletion and the reduction of circulating isoagglutinins, accommodation plays a central role in graft survival in the face of anti-donor (recipient) antibodies [20]. Accommodation may result from one or more of the three following processes: i) a change in antigen, leading to decreased in antibody binding; ii) a change in antibodies, reducing their cytotoxicity; and iii) a change in the graft, enabling it to resist humoral injury [21]. During clinical transplantation accommodation, these processes could manifest as i) temporal inhibition of glycosyltransferase production, resulting in decreased antigenic activity [14]; ii) IgG subclass response [22]; or iii) acquired resistance to complement-mediated lysis [23]. As demonstrated by Tabata et al [24] in their xenograft model, accommodation is thought to be prevalent post-transplantation, with rapid initiation and subsequent persistence for several days. This hypothesis is partially supported by findings demonstrating that onset of acute rejection tends to occur within one month post-transplantation, with a particularly high frequency within the first week [14].
Donor secretor status was determined by genotyping the FUT2, which is known to be phenotypically associated with ABH substance secretion [25]. Previous studies have reported a frequency of 75.5% secretors and 24.5% nonsecretors in the Korean population [26]. We observed a similar frequency in the donor secretor status in our study: 25 donors (78.1%) were secretors, and seven donors (21.9%) were nonsecretors. In addition, we did not observe nonsense mutations at c.428G>A or fusion gene polymorphisms, consistent with previous findings in the Korean population [27].
Most patients experienced one or two titer elevations post-transplantation that did not significantly related to donor secretor status. However, we observed four cases of IgG titer reduction and 10 cases of IgM titer reduction, similar to that observed by Takahashi [14] in patients demonstrating good progress. These titer reductions may be caused by the dilution effect due to the expansion of total body volume post-surgery, neutralization by secreted antigens, or binding and adsorption by the graft. However, since what we measure in the serum is the circulating form of IgM post-transplantation, we assumed that allograft binding and adsorption may be involved in titer reduction. These findings were supported by our observation that titer reduction occurred before elevation post-transplantation, indicating that antibodies were removed from circulation prior to numerical expansion.
Interestingly, IgM titers showed rapid reductions in recipients transplanted with kidneys from RNSD (P=0.01), which could be explained by the lack of absorption effect of soluble antigens that enhance the binding of antibodies to antigens in the allograft. However, the reduction in IgG titers was not significantly different between groups, since anti-A/B antibodies are predominantly of the IgM class and may be more affected by absorption of soluble ABH than IgG owing to its pentamer structure [28].
The biopsies performed in 13 patients with suspected graft rejection showed abnormal findings. ABMR occurs primarily because of the antigen-antibody reaction and therefore causes injury in renal blood vessels through an indirect pathway. In contrast, TCMR is initiated via a direct pathway, resulting in subsequent damage to renal tubules [29]. The presence of soluble antigens enables possible evasion of antibody binding; however, the graft may be endangered by antibody reactions via stimulation of the B-cell response. We predicted that the incidence of ABMR might differ between the two groups but that this difference would not be statistically significant, possibly owing to the small number of patients enrolled in the study. However, ABMR severity was different in two patients; one patient in the RNSD group was stage III, and another patient in the RSD group was stage I. Although more evidence is needed, our findings suggested that the vasculature of grafts from RNSD might be more at risk of ABMR by binding of anti-A/B antibodies due to the lack of protection by soluble ABH antigens.
Interestingly, soluble ABH antigens did not affect rejection-free graft survival; these findings could be explained by the nature of β-galactoside α-1,2-fucosyltransferase, i.e., that some soluble substance protects the graft during the initial phase, with a subsequent decrease in activity and production [3031]. Indeed, during the process of accommodation, protectable soluble ABH is altered, which enabled the graft to resist humoral injury; however, ABH antigenicity has an insignificant role later which leads to a decreased antibody binding.
The main limitations of our study were the small population size, which hindered our evaluation of the statistical significance of the clinical outcomes, and the absence of biopsy protocols, which could have provided additional histological information regarding the grafts. Furthermore, antibody titers can be quantified by using advanced methodologies, such as flow cytometry or immunoassays, which could have yielded more accurate correlations [32]. However, further studies with larger cohorts are required to elucidate the influence of ABH secretor status on long-term outcomes.
In conclusion, the secretor status of donor may have an influence on early accommodation in patients undergoing ABOi KT. However, this could not be supported by anti-A/B antibody titers, renal function, or graft survival. The minute observations on protocol biopsies and quantification of antigens and antibodies with sensitive techniques are needed in larger prospective studies of long-term follow-up.
References
1. Nelson PW, Shield CF 3rd, Muruve NA, Murillo D, Warady BA, Aeder MI, et al. Increased access to transplantation for blood group B cadaveric waiting list candidates by using A2 kidneys: time for a new national system? Am J Transplant. 2002; 2:94–99. PMID: 12095063.
2. Porter KA. Morphological aspects of renal homograft rejection. Br Med Bull. 1965; 21:171–175. PMID: 14313592.
3. Yoo S, Lee EY, Huh KH, Kim MS, Kim YS, Kim HO. Role of plasma exchange in ABO-incompatible kidney transplantation. Ann Lab Med. 2012; 32:283–288. PMID: 22779070.
4. Cosio FG, Lager DJ, Lorenz EC, Amer H, Gloor JM, Stegall MD. Significance and implications of capillaritis during acute rejection of kidney allografts. Transplantation. 2010; 89:1088–1094. PMID: 20216485.
5. Tantravahi J, Womer KL, Kaplan B. Why hasn't eliminating acute rejection improved graft survival? Annu Rev Med. 2007; 58:369–385. PMID: 17002551.
6. Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000; 108:1–28. PMID: 10698081.
7. Oriol R, Cartron JP, Cartron J, Mulet C. Biosynthesis of ABH and Lewis antigens in normal and transplanted kidneys. Transplantation. 1980; 29:184–188. PMID: 6987782.
8. Ulfvin A, Bäcker AE, Clausen H, Hakomori S, Rydberg L, Samuelsson BE, et al. Expression of glycolipid blood group antigens in single human kidneys: change in antigen expression of rejected ABO incompatible kidney grafts. Kidney Int. 1993; 44:1289–1297. PMID: 7508004.
9. Kumazaki T, Yoshida A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci USA. 1984; 81:4193–4197. PMID: 6588382.
10. Sarnesto A, Köhlin T, Hindsgaul O, Vogele K, Blaszczyk-Thurin M, Thurin J. Purification of the β-N-acetylglucosaminide α 1→3-fucosyltransferase from human serum. J Biol Chem. 1992; 267:2745–2752. PMID: 1733970.
11. Wilbrandt R, Tung KS, Deodhar SD, Nakamoto S, Kolff WJ. ABO blood group incompatibility in human renal homotransplantation. Am J Clin Pathol. 1969; 51:15–23. PMID: 4885605.
12. Montgomery RA, Hardy MA, Jordan SC, Racusen LC, Ratner LE, Tyan DB, et al. Consensus opinion from the antibody working group on the diagnosis, reporting, and risk assessment for antibody-mediated rejection and desensitization protocols. Transplantation. 2004; 78:181–185. PMID: 15280674.
13. Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003; 3:952–960. PMID: 12859529.
14. Takahashi K. Accommodation in ABO-incompatible kidney transplantation: why do kidney grafts survive? Transplant Proc. 2004; 36(2 Suppl):193S–196S. PMID: 15041335.
15. Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Hum Immunol. 2007; 68:645–651. PMID: 17678718.
16. Park KU, Song J, Han KS, Kim JQ. The fusion allele of the FUT2 (secretor type α(1,2)-fucosyltransferase) gene at a high frequency and a new se385 allele in a Korean population. Ann Hematol. 2005; 84:656–660. PMID: 15809881.
17. Fung MK, Grossman BJ, editors. Technical manual. 18th ed. Bethesda: American Association of Blood Banks;2014.
18. Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012; 12:563–570. PMID: 22300494.
19. Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000; 6:686–688. PMID: 10835686.
20. Lynch RJ, Platt JL. Accommodation in renal transplantation: unanswered questions. Curr Opin Organ Transplant. 2010; 15:481–485. PMID: 20613524.
21. Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990; 11:450–456. PMID: 2073317.
22. Rydberg L. ABO-incompatibility in solid organ transplantation. Transfus Med. 2001; 11:325–342. PMID: 11532188.
23. Costa C, Zhao L, Burton WV, Rasas C, Bondiol KR, Williams BL, et al. Transgenic pigs designed to express human CD59 and H-transferase to avoid humoral xenograft rejection. Xenotransplantation. 2002; 9:45–57. PMID: 12005104.
24. Tabata T, de Perrot M, Keshavjee S, Liu M, Downey GP, Waddell TK. Accommodation after lung xenografting from hamster to rat. Transplantation. 2003; 75:607–612. PMID: 12640297.
25. Kudo T, Iwasaki H, Nishihara S, Shinya N, Ando T, Narimatsu I, et al. Molecular genetic analysis of the human Lewis histo-blood group system. II. Secretor gene inactivation by a novel single missense mutation A385T in Japanese nonsecretor individuals. J Biol Chem. 1996; 271:9830–9837. PMID: 8621666.
26. Moon CK, Kany YB, Lee DW, Kang DY, Lee D. Lewis phenotypes and ABH secretor status of Korean adults. Korean J Clin Pathol. 1984; 4:237–244.
27. Suh IB, Hwang MW, Lim CS, Lee CK, Ma KR, Kim YK, et al. The genotyping of the secretory gene (Sec2) in the Korean population. Korean J Clin Pathol. 1999; 19:572–577.
28. Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005; 130:954–963. PMID: 16156865.
29. Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2014; 85:258–264. PMID: 23965521.
30. Yoshida A, Schmidt GM, Blume KG, Beutler E. Plasma blood group glycosyltransferase activities after bone marrow transplantation. Blood. 1980; 55:699–701. PMID: 6766757.
31. Takahashi K. A new concept of accommodation in ABO-incompatible kidney transplantation. Clin Transplant. 2005; 19(Suppl 14):76–85. PMID: 15955174.
32. Won DI, Jung OJ, Lee YS, Kim SG, Suh JS. Flow cytometry antibody screening using pooled red cells. Cytometry B Clin Cytom. 2010; 78:96–104. PMID: 19714726.
Fig. 1
The time (days) required for onefold elevation of IgG and IgM and reduction of IgG after transplant according to recipient groups. The box plot shows the median (bold line), the first quartile (lower border of the box) and the third quartile (upper border of the box).
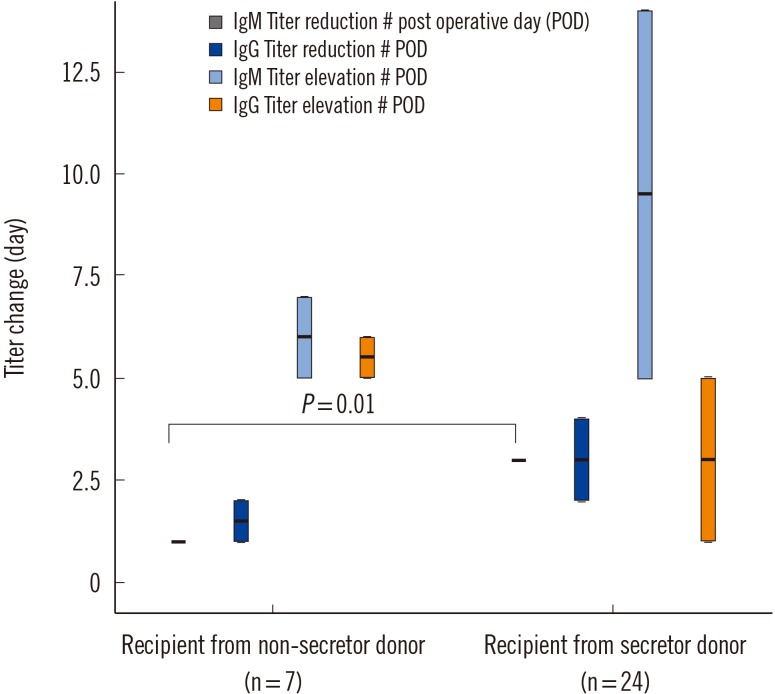
Fig. 2
Rejection-free graft survival within one month post-transplantation (A) and within one year post-transplantation between groups (B). The differences in survival rates were not significant at both time points (95% confidence interval, 27.8–31.6, P=0.960 for one month; 286.4–359.8, P=0.943 for one year).
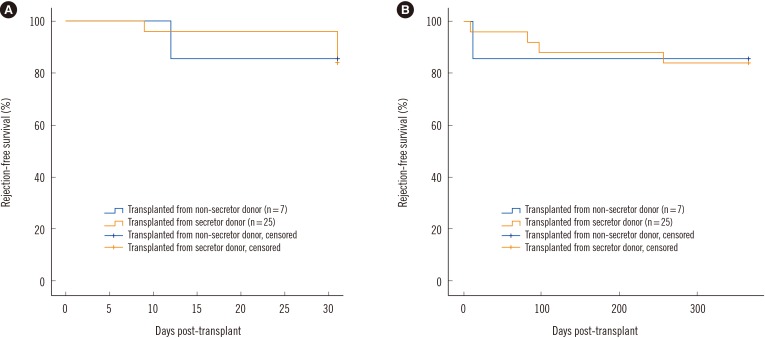
Table 1
Demographic data of recipients
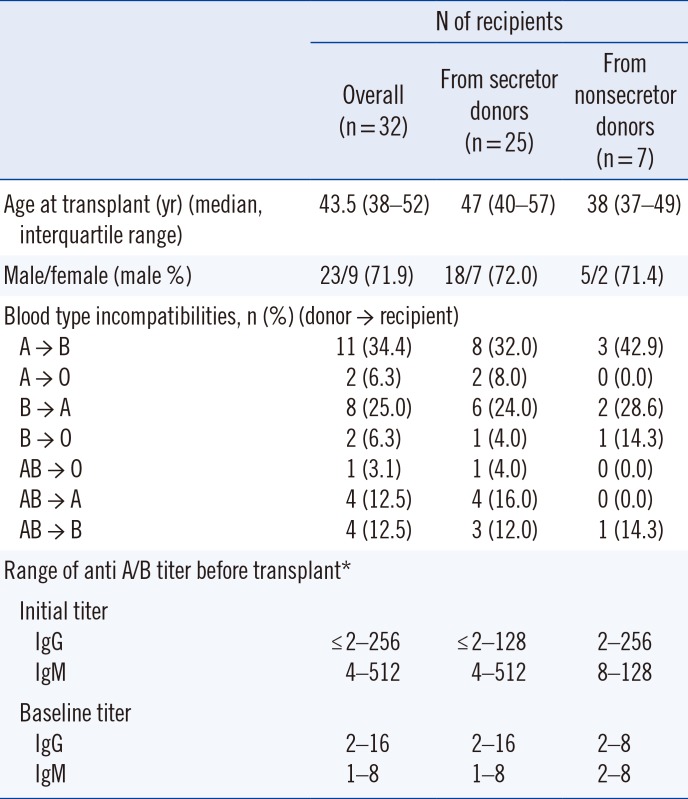
Table 2
Distribution of secretor genotypes in 32 donors
| Estimated phenotype | N (%) | |
|---|---|---|
| Se357/se357,385 | Secretor | 15 (46.9) |
| Se/se357,385 | 6 (18.8) | |
| Se357/Se357 | 3 (9.4) | |
| Se/Se357 | 1 (3.1) | |
| se357,385/se357,385 | Nonsecretor | 7 (21.9) |
| Total | 32 (100.0) |
Table 3
Clinical outcomes of patients according to donors' secretor status
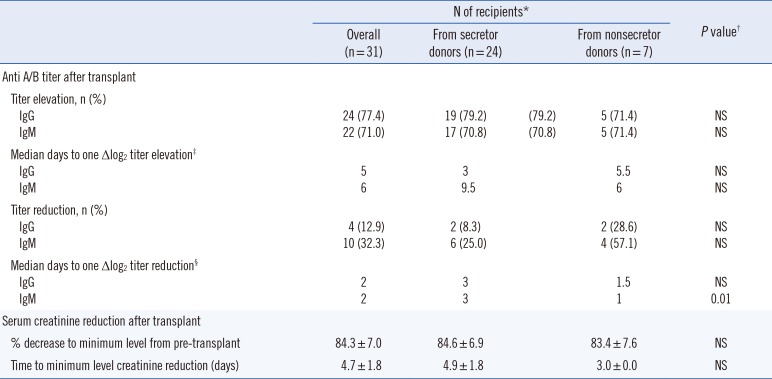
*One patient who received a kidney from a secretor donor was excluded; †Pearson chi-square tests and Fisher's exact tests were used to compare nominal variables, and Mann-Whitney U tests were used for continuous variables to compare clinical outcomes of patients according to donors' secretor status; ‡Statistical analyses of IgG and IgM elevations were available in 25 and 23 patients, respectively; §Statistical analyses of IgG and IgM reductions were available in 4 and 10 patients, respectively.
Abbreviation: NS, not significant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download