Abstract
Background
National surveillance of antimicrobial resistance becomes more important for the control of antimicrobial resistance and determination of treatment guidelines. We analyzed Korean Antimicrobial Resistance Monitoring System (KARMS) data collected from 2013 to 2015.
Methods
Of the KARMS participants, 16 secondary or tertiary hospitals consecutively reported antimicrobial resistance rates from 2013 to 2015. Data from duplicate isolates and institutions with fewer than 20 isolates were excluded. To determine the long-term trends, previous KARMS data from 2004 to 2012 were also considered.
Results
The prevalence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium from 2013 to 2015 was 66–72% and 29–31%, respectively. The resistance rates of Escherichia coli to cefotaxime and cefepime gradually increased to 35% and 31%, respectively, and fluoroquinolone resistance reached 48% in 2015. The resistance rates of Klebsiella pneumoniae to cefotaxime, cefepime, and carbapenem were 38–41%, 33–41%, and <0.1–2%, respectively, from 2013 to 2015. The carbapenem susceptibility rates of E. coli and K. pneumoniae decreased from 100% and 99.3% in 2011 to 99.0% and 97.0% in 2015, respectively. The resistance rate of Pseudomonas aeruginosa to carbapenem increased to 35% and the prevalence of carbapenem-resistant Acinetobacter baumannii increased from 77% in 2013 to 85% in 2015.
Conclusions
Between 2013 and 2015, the resistance rates of E. coli to third- and fourth-generation cephalosporins increased continuously, while carbapenem-susceptibility gradually decreased, particularly in K. pneumoniae. The prevalence of carbapenem-resistant P. aeruginosa and A. baumannii increased significantly; therefore, few treatment options remain for these resistant strains.
The prevalence of antimicrobial-resistant bacteria has increased worldwide. In 2013, the Centers for Disease Control and Prevention in the United States reported that each year, at least two million people in the United States acquire serious infections due to resistant bacteria and that over 23,000 people die as a direct result of these antibiotic-resistant infections [1]. Resistance rates vary across countries because of differences in antimicrobial agent usage and systems for the prevention of antimicrobial resistant bacteria. In addition to resistance rates, modes of resistance also differ among countries and even among cities within the same country. Therefore, accurate nationwide surveillance of antimicrobial-resistant bacteria is becoming more important for the establishment of treatment guidelines for optimal applied therapies.
The spread of antimicrobial resistance is a major concern in Korea. In 2003, the prevalence of resistance in bacterial strains included 66% methicillin-resistant Staphylococcus aureus (MRSA), 22% vancomycin-resistant Enterococcus faecium, 14% ceftazidime-resistant Klebsiella pneumoniae, and 25% imipenem-resistant Pseudomonas aeruginosa [2]. Lee et al [3] reported that according to the Korean Nationwide Surveillance of Antimicrobial Resistance (KONSAR) study, the prevalence of ceftazidime-resistant K. pneumoniae and imipenem-resistant Acinetobacter species increased up to 47% and 22%, respectively. In 2009, the prevalence of imipenem-resistant Acinetobacter species increased to 57% [4]. Recently, carbapenem-resistant Enterobacteriaceae (CRE) have been increasingly reported in many hospitals in Korea, mostly associated with the clonal expansion of K. pneumoniae carbapenemase [5]. The Korean government has implicated six multidrug-resistant organisms, including MRSA, vancomycin-resistant Staphylococcus aureus (VRSA), vancomycin-resistant enterococci, multidrug-resistant P. aeruginosa, and multidrug-resistant Acinetobacter baumannii (MRAB), as the main agents of nosocomial infections. Since 2012, Korean hospitals are required to report infections by these organisms to the Korea Centers for Disease Control and Prevention (http://www.cdc.go.kr/CDC/). However, this program focuses solely on antimicrobial resistance rates and not on particular strain differences; further evaluation for elucidating the mode of resistance is not available through this program.
The Korean Antimicrobial Resistance Monitoring System (KARMS), which has been overseen by the Korea Centers for Disease Control and Prevention since 2002, is a surveillance program for collecting antimicrobial resistance data in Korea as well as a limited number of resistant strain isolates. A total of 35 tertiary and secondary hospitals participate in this program by reporting the antimicrobial resistance rates of various bacteria once a year. In this study, we evaluated the antimicrobial resistance rates of the most commonly isolated bacteria, including S. aureus, Enterococcus species, Streptococcus pneumoniae, Escherichia coli, K. pneumoniae, P. aeruginosa, and A. baumannii, on the basis of the data from 16 hospitals that continuously reported to KARMS between 2013 and 2015.
Antimicrobial resistance data was collected from 31 secondary or tertiary hospitals; 16 of these hospitals reported annually from 2013 to 2015. These 16 hospitals (620–1,356 beds) covered eight major cities and provinces in Korea, representing national antimicrobial resistance trends. The hospitals used their own hospital system or WHONET software [6] and duplicate isolates were excluded from the analysis. Antimicrobial susceptibility tests were performed by using disk diffusion or modified broth microdilution tests with the VITEK 2 automated system (bioMérieux, Marcy l'Etoile, France) or the MicroScan system (Beckman Coulter, Brea, CA, USA) according to the CLSI guidelines [7]. If the CLSI guideline threshold was not available, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) threshold was applied for the determination of susceptibility. To minimize size difference bias between the hospitals, data consisting of less than 20 isolates of an organism were excluded; resistance rates were calculated as the median and range because of the limited number of hospitals. The antimicrobial resistance of gram-negative bacilli was compared with previous results from 2004 to determine long-term trend changes [8910].
The resistance rates of gram-positive cocci from 2013 to 2015 are summarized in Table 1. The prevalence of MRSA decreased from 72% in 2013 to 66% in 2015. The resistance rates of S. aureus isolates to other antibiotics also slightly decreased from 2013; 95% for penicillin, 54% for erythromycin, 54% for clindamycin, and 48% for ciprofloxacin in 2015. Over 97% of the isolates showed susceptibility to trimethoprim-sulfamethoxazole, while no vancomycin- and/or teicoplanin-resistant S. aureus were isolated. The rates of penicillin resistance in S. pneumoniae ranged from 11–14%; and 9–12% of isolates were resistant to levofloxacin. In Enterococcus faecalis, ampicillin resistance was low (0–2%); however, 61% and 18% of isolates were resistant to high-level gentamicin (minimum inhibitory concentration [MIC] >500 µg/mL) and high-level streptomycin (MIC >1,000 µg/mL), respectively. The rates of vancomycin resistance were 0.8–1% in both 2013 and 2015. In contrast, ampicillin resistance was widespread in E. faecium (93–94%) and 29–31% of isolates exhibited vancomycin resistance during the test period.
The antimicrobial resistance rates of Enterobacteriaceae are detailed in Table 2. The resistance rates of E. coli isolates were 69–72% to ampicillin, 29–35% for cefotaxime, 30–31% for ceftazidime, 28–31% for cefepime, and 9–10% for cefoxitin from 2013 to 2015, respectively. The resistance rates to fluoroquinolone increased from 42% in 2013 to 48% in 2015, while the resistance rate to trimethoprim-sulfamethoxazole remained stable at 38–39%. In K. pneumoniae, the resistance rates to third- and fourth-generation cephalosporins were 36–41% and 33–41%, respectively. The resistance rates were 12–17% for cefoxitin, 34–35% for fluoroquinolone, and 21–30% for trimethoprim-sulfamethoxazole. Over the last decade, the rates of resistance to the third- and fourth-generation cephalosporins and fluoroquinolone have steadily increased, except resistance to cefoxitin, which decreased significantly (Fig. 1). The resistance rate to carbapenems in E. coli and K. pneumoniae was 0.1% in 2013 and 0.1% and 1%, respectively, in 2015. However, the susceptibility rates in both species decreased from 99.7% and 98.7% in 2013 to 99.3% and 97.0% in 2015, respectively (Fig. 2).
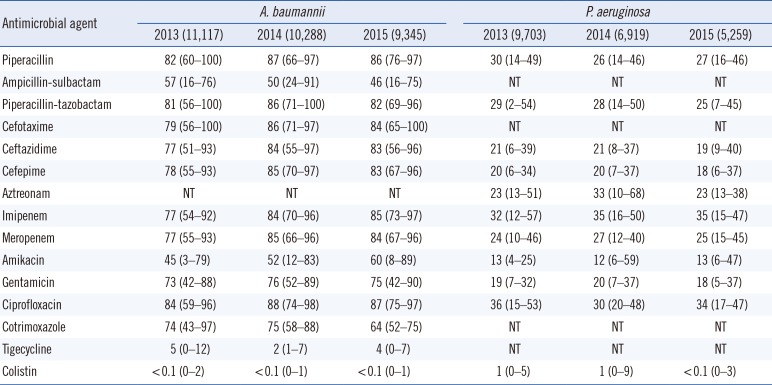
The antimicrobial resistance rates of A. baumannii and P. aeruginosa are shown in Table 3. The resistance rates of A. baumannii were 77–86% for extended-spectrum cephalosporins, 77–85% for carbapenems, 84–88% for fluoroquinolone, 45–60% for amikacin, and 73–76% for gentamicin. The resistance rate to ampicillin-sulbactam decreased from 57% in 2013 to 46% in 2015. The resistance rates were 2–5% for tigecycline and less than 0.1% for colistin. In P. aeruginosa, the resistance rates to ceftazidime, cefepime, amikacin, and gentamicin were 19–21%, 18–20%, 12–13%, and 18–20%, respectively. The resistance rate of carbapenem-resistant P. aeruginosa from 2013 to 2015 was 32–35% for imipenem and 24–27% for meropenem (Fig. 3). The resistance rates of A. baumannii showed a steady increase, except for ampicillin-sulbactam, while the resistance rates of P. aeruginosa exhibited a decrease, except for imipenem.
The prevalence of antimicrobial-resistant organisms varies greatly among hospitals and countries. Moreover, changes in resistance rates differ greatly depending on time and region. For example, Scandinavian countries, such as Sweden, show low MRSA levels (<1%) [11]. However, the rates of MRSA in other European countries, Japan, and Korea are as high as 40–60% [412]. Therefore, national surveillance systems are becoming more important for guiding clinicians in terms of empirical treatment for suspected infections and the control of antimicrobial resistance dissemination. KARMS data allowed us to analyze national trends of antimicrobial resistance and detect the emergence of new resistances in Korea.
MRSA is the one of the most important resistant pathogens; we found that the rate of MRSA slightly decreased from a peak of 75% in 2009 to 66% (55% in blood isolates, data not shown) in 2015 [4]. The prevalence of MRSA infection could be affected by carriage rate and healthcare-associated infection control; in addition, this decrease might be due to the introduction of the decolonization and hand-hygiene program (the most common transmission route of MRSA [13]) for controlling infection in healthcare-associated settings in Korea [14]. Vancomycin and teicoplanin are drugs commonly used for MRSA treatment; however, none of the S. aureus isolates showed vancomycin resistance between 2013 and 2015 and most isolates exhibited susceptibility to linezolid.
In S. pneumoniae, the resistance rate to penicillin G ranged from 11% to 14% during 2014 and 2015, which is similar to previous results following the application of a new breakpoint [15]. However, the resistance rate to erythromycin remained high (>80%) in 2015. A recent survey of the empirical treatment of community-acquired pneumonia in Korea reported that 13.4% of local clinic doctors still prescribe macrolide monotherapy [16]; increased resistance to erythromycin could be the result of treatment failure due to this regimen. The resistance rate to fluoroquinolone was as low as 9–12%; however, this did represent an increase from the 2–5% reported in 2005 and 2006 [17]. Previous use of fluoroquinolones, cerebrovascular disease, and healthcare-associated infection have been significantly associated with levofloxacin resistance in S. pneumoniae [18]; thus, antimicrobial stewardship and an infection control program are required to prevent the spread of fluoroquinolone-resistance.
Resistance rates varied among Enterococcus species. In E. faecalis, the resistance rates were extremely low, 0–2% to ampicillin and 1% to vancomycin, which is consistent with previous results since 2004 [8910]. E. faecium showed a high prevalence of ampicillin resistance (> 90%) and the resistance rates to vancomycin and teicoplanin were 30% and 26%, respectively. The similarity in the resistance rates to teicoplanin and vancomycin might indicate that most isolates contain vanA gene clusters; the small discrepancy could be related to the presence of the vanA gene with a VanB phenotype [19]. The resistance rates of E. faecium to linezolid and quinupristin/dalfopristin did not increase during the surveillance period. The linezolid susceptibility of most isolates can be explained by the fact that the most common mechanism of linezolid resistance is a mutation in the linezolid binding site, which cannot be spread by horizontal transfer, in contrast to the acquisition of the cfr gene. Persistent daptomycin susceptibility (data not shown) might be caused by the unavailability of this antimicrobial agent in Korea. Indeed, increased minimal inhibitory concentrations of daptomycin have been associated with treatment failure in other countries [20].
Resistance to third- and fourth-generation cephalosporins in E. coli has increased since 2004 (Fig. 1). The resistance rate to cefotaxime was slightly higher than that to ceftazidime, which is consistent with a previous report on the dissemination of CTX-M type extended spectrum β-lactamase (ESBL) rather than TEM- or SHV- type ESBL [21]. As fluoroquinolone-resistant E. coli also increased to 48%, the empirical use of fluoroquinolone for urinary tract infections caused primarily by E. coli should be avoided, as previously reported [22]. The reason underlying the continual increase in fluoroquinolone resistance is unclear; it might be related to mutation of the gyrase or topoisomerase IV genes including qyrA, gyrB, parC and parE [23]. Moreover, the spread of plasmid-mediated fluoroquinolone-resistance, such as the qnr gene, should also be considered [24]. Further evaluations are required to determine the mode of resistance. Interestingly, only the resistance rate to cefoxitin decreased to 9%. This finding might indicate the reduced prevalence of plasmid-mediated AmpC β-lactamase genes, such as DHA-1 and CMY-1 [25], due to lowered selective pressure for cephamycin. In K. pneumoniae, the resistance rates to most antimicrobial agents, including third- and fourth-generation cephalosporins and fluoroquinolone, remained similar to previous results, except for decreased resistance to cefoxitin. The rates in CRE remained at <1% in 2015; however, the susceptibility rates to carbapenem (especially meropenem) in Enterobacteriaceae, including E. coli and K. pneumoniae, decreased from 100% and 99.3 in 2011 to 99.0% and 97.0% in 2015, respectively. Furthermore, carbapenem non-susceptible E. coli were reported in 75.0% of hospitals in 2015, which was higher than in 2013 (43.8%), and carbapenem non-susceptible K. pneumoniae were reported in all participating hospitals from 2013 to 2015. Therefore, appropriate surveillance using rapid detection and infection control strategies for CRE should be mandated to prevent the spread of CRE, especially carbapenemase-producing organisms. Furthermore, molecular genetic studies of multidrug-resistant Enterobacteriaceae should be conducted to elucidate the mechanisms of resistance and dissemination.
P. aeruginosa and A. baumannii are important pathogens in healthcare-associated infections. The emergence of multidrug-resistant P. aeruginosa and multidrug-resistant A. baumannii is a major concern in Korea, because few drugs, except for polymyxins, are available for the treatment of these infections. The incidence of carbapenem-resistant P. aeruginosa was 35% in 2015, higher than the rate reported by Lee et al in 2009 (23%) [4]. Surprisingly, the resistance rates of P. aeruginosa to third- and fourth-generation cephalosporins, amikacin, and gentamicin decreased significantly with the increased resistance rate to carbapenem, similar to observations in France from the last 4 years; however, the mechanism underlying this phenomenon remains unclear [11]. Another remarkable change in P. aeruginosa carbapenem resistance is the emergence of ST235 bearing the IMP-6 metallo-β-lactamase since the early 2010s. Although the reason for the transition from the VIM-2 to IMP-6 phenotype is not clear [2627], the increased use of meropenem may be associated with the dissemination of IMP-6, because IMP-6 can hydrolyze meropenem more efficiently than VIM-2 [28]. In addition, the rate of carbapenem-resistant A. baumannii gradually increased to >80%. This finding can be explained by the horizontal spread of A. baumannii carrying the blaOXA-23-like gene, which is the most common type of carbapenem resistance in Korea [29]. In contrast, the resistance rate to ampicillin-sulbactam decreased to 46% in 2015. Therefore, ampicillin-sulbactam could constitute a treatment option for MRAB in combination with colistin [30]. The resistance rate of A. baumannii to tigecycline remained at <5% in 2015, when the breakpoint for Enterobacteriaceae was applied. However, several studies have demonstrated the inefficacy of tigecycline for the treatment of MRAB because of rapid distribution to the tissue, which results in increased all-cause mortality [31].
In conclusion, we analyzed KARMS data from 16 hospitals from 2013 to 2015 with respect to antimicrobial resistance trends. The resistance rate of E. coli to third- and fourth-generation cephalosporins and fluoroquinolone increased significantly and carbapenem-non-susceptible Enterobacteriaceae are becoming a more common clinical isolate. The prevalence of carbapenem-resistant P. aeruginosa and A. baumannii increased significantly, leaving few remaining treatment options for these infections. Furthermore, nationwide antimicrobial susceptibility surveillance should be improved in order to standardize the quality of data and meet global needs.
Acknowledgments
The authors wish to thank to the officers of the department of drug resistance at the Korea Centers for Disease Control and Prevention (KCDC) and other participants of the KARMS program.
References
1. Centers for Disease Control and Prevention. Antibiotic resistance threat in the United States, 2013. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Service;2013.
2. Hong SG, Yong D, Lee K, Kim EC, Lee WK, Jeong SH, et al. Antimicrobial resistance of clinically important bacteria isolated from hospitals located in representative provinces of Korea. Korean J Clin Microbiol. 2003; 6:29–36.
3. Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S, et al. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J. 2010; 51:901–911. PMID: 20879058.
4. Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, et al. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011; 52:793–802. PMID: 21786445.
5. Lee Y, Kim BS, Chun J, Yong JH, Lee YS, Yoo JS, et al. Clonality and resistome analysis of KPC-producing Klebsiella pneumoniae strain isolated in Korea using whole genome sequencing. Biomed Res Int. 2014; 2014:352862. PMID: 25105122.
6. O'Brien TF, Stelling JM. WHONET: removing obstacles to the full use of information about antimicrobial resistance. Diagn Microbiol Infect Dis. 1996; 25:162–168. PMID: 8937840.
7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty fourth Informational supplement, M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute;2014.
8. Lee H, Yong D, Lee K, Hong SG, Kim EC, Jeong SH, et al. Antimicrobial resistance of clinically important bacteria from 12 hospitals in Korea in 2004. Korean J Clin Microbiol. 2005; 8:66–73.
9. Lee H, Kim CK, Lee J, Lee SH, Ahn JY, Hong SG, et al. Antimicrobial resistance of clinically important bacteria isolated from 12 hospitals in Korea in 2005 and 2006. Korean J Clin Microbiol. 2007; 10:59–69.
10. Shibayama K, Lee H, Kim S. Comparison of antibiotic resistance rate of medically important microorganisms between Japan and Korea. Ann Clin Microbiol. 2015; 18:111–118.
11. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Solna, Sweden: 2015;http://ecdc.europa.eu/en/.
12. Ministry of Health, Labour and Welfare. Japan Nosocomial Infections Surveillance (JANIS); Annual Open Report. 2014. http://www.nih-janis.jp/.
13. Barnes SL, Morgan DJ, Harris AD, Carling PC, Thom KA. Preventing the transmission of multidrug-resistant organisms: modeling the relative importance of hand hygiene and environmental cleaning interventions. Infect Control Hosp Epidemiol. 2014; 35:1156–1162. PMID: 25111924.
14. Kim MK, Nam EY, Na SH, Shin MJ, Lee HS, Kim NH, et al. Discrepancy in perceptions regarding patient participation in hand hygiene between patients and health care workers. Am J Infect Control. 2015; 43:510–515. PMID: 25752956.
15. Choi SH, Chung JW, Sung H, Kim MN, Kim SH, Lee SO, et al. Impact of penicillin nonsusceptibility on clinical outcomes of patients with nonmeningeal Streptococcus pneumoniae bacteremia in the era of the 2008 clinical and laboratory standards institute penicillin breakpoints. Antimicrob Agents Chemother. 2012; 56:4650–4655. PMID: 22687517.
16. Kim HI, Kim SW, Chang HH, Lee JM, Peck KR. A 2011-2012 survey of doctors' perceptions of korean guidelines and empirical treatment of community-acquired pneumonia. Infect Chemother. 2013; 45:394–405. PMID: 24475353.
17. Park KS, Kim MH, Park TS, Suh JT, Lee HJ. Antimicrobial resistance of enterococcal isolates from blood and risk factors for vancomycin resistant enterococcal bacteremia in a tertiary care university hospital from 2003 to 2007. Korean J Clin Microbiol. 2010; 13:59–67.
18. Kang CI, Song JH, Kim SH, Chung DR, Peck KR, So TM, et al. Risk factors for levofloxacin-nonsusceptible Streptococcus pneumoniae in community-acquired pneumococcal pneumonia: a nested case-control study. Eur J Clin Microbiol Infect Dis. 2014; 33:55–59. PMID: 24062235.
19. Jung MK, Ahn SH, Lee WG, Lee EH. Molecular epidemiology of vancomycin-resistant enterococci isolated from non-tertiary-care and tertiary-care hospitals in Korea. Epidemiol Infect. 2014; 142:2372–2377. PMID: 25267406.
20. Boucher HW, Sakoulas G. Perspectives on Daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis. 2007; 45:601–608. PMID: 17682996.
21. Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, et al. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect Dis. 2012; 12:149. PMID: 22747570.
22. Jeon JH, Kim K, Han WD, Song SH, Park KU, Rhee JE, et al. Empirical use of ciprofloxacin for acute uncomplicated pyelonephritis caused by Escherichia coli in communities where the prevalence of fluoroquinolone resistance is high. Antimicrob Agents Chemother. 2012; 56:3043–3046. PMID: 22391544.
23. Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005; 41(S2):S120–S126. PMID: 15942878.
24. Yang HY, Nam YS, Lee HJ. Prevalence of plasmid-mediated quinolone resistance genes among ciprofloxacin-nonsusceptible Escherichia coli and Klebsiella pneumonia isolated from blood cultures in Korea. Can J Infect Dis Med Microbiol. 2014; 25:163–169. PMID: 25285114.
25. Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, et al. Prevalence of plasmid-mediated AmpC beta-lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb Drug Resist. 2006; 12:44–49. PMID: 16584308.
26. Seok Y, Bae IK, Jeong SH, Kim SH, Lee H, Lee K. Dissemination of IMP-6 metallo-β-lactamase-producing Pseudomonas aeruginosa sequence type 235 in Korea. J Antimicrob Chemother. 2011; 66:2791–2796. PMID: 21933788.
27. Yong D, Choi YS, Roh KH, Kim CK, Park YH, Yum JH, et al. Increasing prevalence and diversity of metallo-beta-lactamases in Pseudomonas spp., Acinetobacter spp., and Enterobacteriaceae from Korea. Antimicrob Agents Chemother. 2006; 50:1884–1886. PMID: 16641469.
28. Yano H, Kuga A, Okamoto R, Kitasato H, Kobayashi T, Inoue M. Plasmid-encoded metallo-beta-lactamase (IMP-6) conferring resistance to carbapenems, especially meropenem. Antimicrob Agents Chemother. 2001; 45:1343–1348. PMID: 11302793.
29. Lee Y, Kim YR, Kim J, Park YJ, Song W, Shin JH, et al. Increasing prevalence of blaOXA-23-carrying Acinetobacter baumannii and the emergence of blaOXA-182-carrying Acinetobacter nosocomialis in Korea. Diagn Microbiol Infect Dis. 2013; 77:160–163. PMID: 23891219.
30. Kuo LC, Lai CC, Liao CH, Hsu CK, Chang YL, Chang CY, et al. Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin Microbiol Infect. 2007; 13:196–198. PMID: 17328733.
31. Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012; 54:1699–1709. PMID: 22467668.
Fig. 1
Antimicrobial resistance rates (%) of Escherichia coli and Klebsiella pneumoniae from 2004 to 2015. The resistance rates from 2004 to 2012 were from the previous publication of the KARMS [8910]. The resistance rates of E. coli to fluoroquinolone, third- and fourth-generation cephalosporins (CAZ, CTX, FEP) gradually increased since 2004. However, the resistance rates of E. coli and K. pneumonia to cephamycin decreased. The vertical dotted lines indicate the application of the lowered cefotaxime and ceftazidime breakpoints according to changes in the CLSI guideline in 2010.
Abbreviations: CAZ, ceftazidime; CTX, cefotaxime; FEP, cefepime; FOX, cefoxitin; FQ, fluoroquinolone.
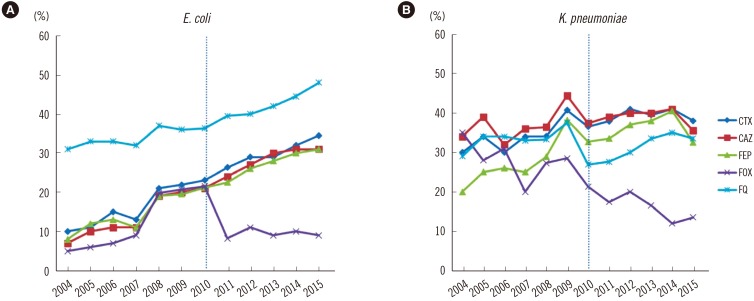
Fig. 2
Carbapenem susceptibility rates of Escherichia coli and Klebsiella pneumoniae from 2011 to 2015. The resistance rates from 2011 to 2012 were from the previous publication of the KARMS [10].
Abbreviations: ECO, E. coli; KPN, K. pneumoniae; S, susceptible.
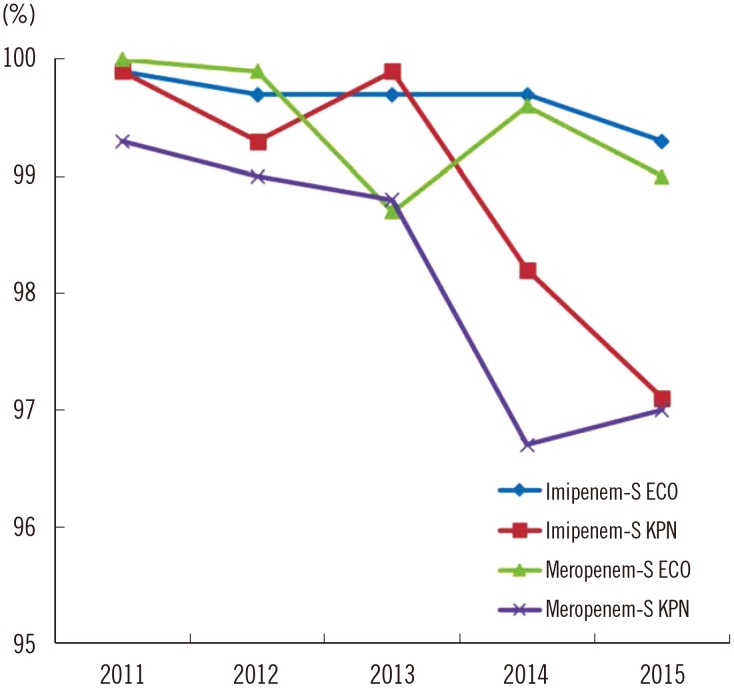
Fig. 3
Antimicrobial resistance rates (%) of Acinetobacter baumannii and Pseudomonas aeruginosa from 2004 to 2015. The resistance rates from 2004 to 2012 were from the previous publication of the KARMS [8910].
Abbreviations: CAZ, ceftazidime; FEP, cefepime; FQ, fluoroquinolone; IMP, imipenem; MEM, meropenem; Amp-Sul, ampicillin-sulbactam.
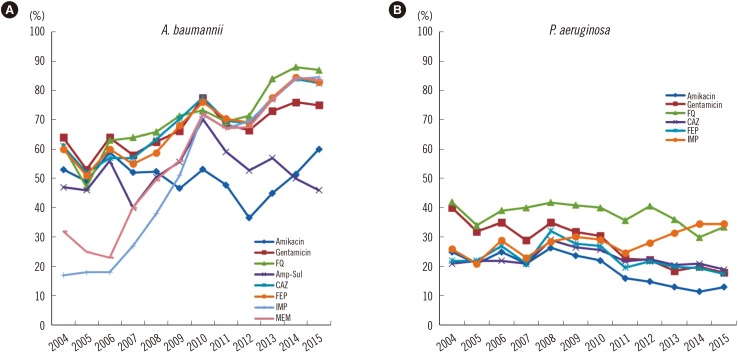
Table 1
Antimicrobial resistance rates (%) of gram-positive cocci
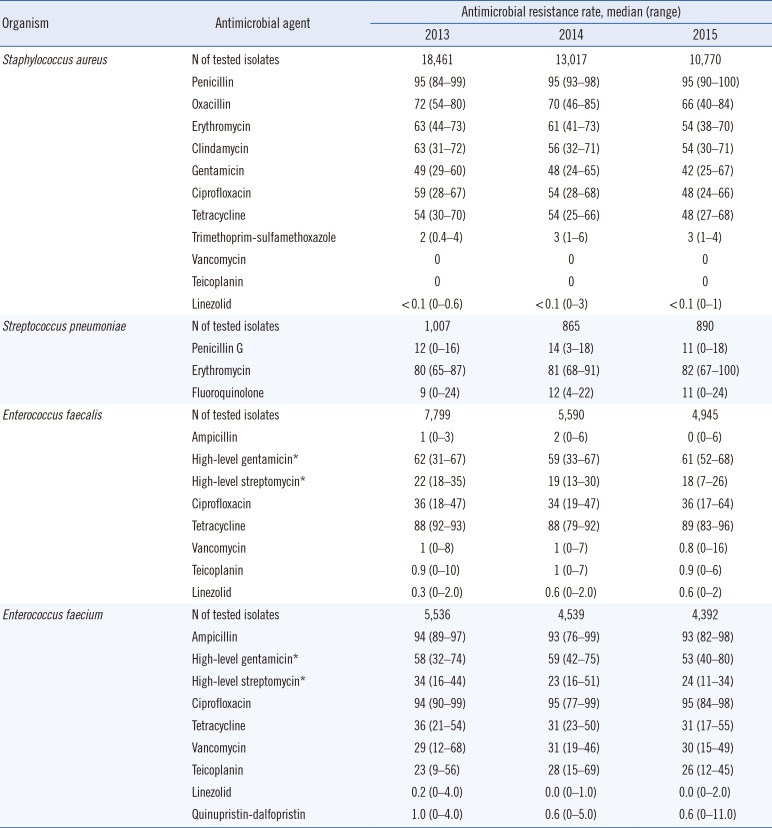
Table 2
Antimicrobial resistance rates (%) of Escherichia coli and Klebsiella pneumoniae in Korea from 2013 to 2015*
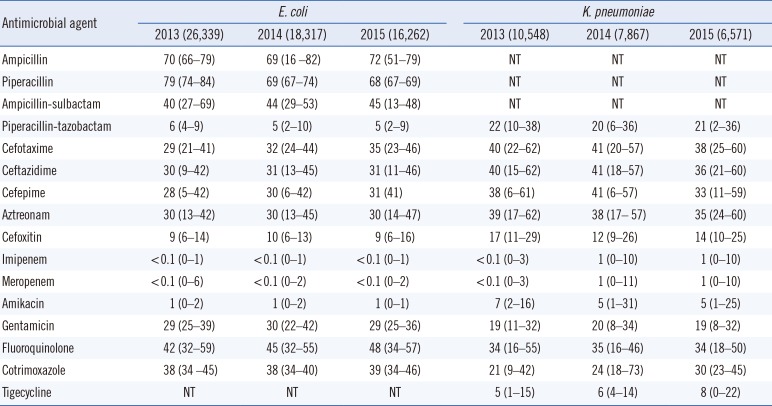
Table 3
Antimicrobial resistance rates (%) of Acinetobacter baumannii and Pseudomonas aeruginosa in Korea from 2013 to 2015*




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download