Dear Editor,
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory disorder that is characterized by fever, hepatosplenomegaly, and cytopenia. Primary HLH is an autosomal recessive disease and appears in infants or young children, whereas secondary HLH affects older children or adults who do not have a family history of similar conditions or a confirmed genetic disorder. Known gene mutations and their associated familial HLH (FHL) subtypes are as follows: PRF1 with FHL2, UNC13D with FHL3, STX11 with FHL4, and most recently, STXBP2 with FHL5 [1]. Here, we report a rare case of HLH with a novel PRF1 mutation.
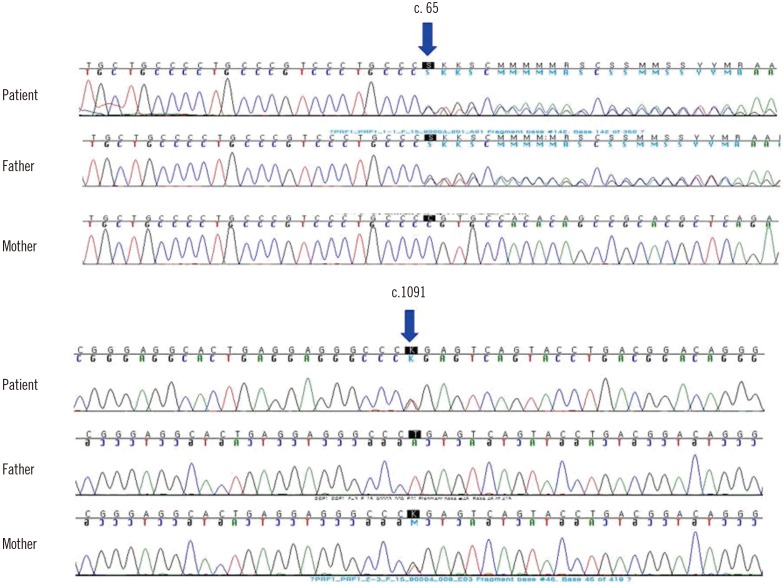
A two-month-old female was admitted because of a one-week history of fever and cytopenia that did not respond to antipyretics or empirical antibiotic therapy. She was born at 39+6 weeks of gestation with a birth weight of 3.5 kg. She had no siblings, and her family history was unremarkable. At admission, the patient's temperature was greater than 38.5℃ and splenomegaly was present (>3 fingers). Laboratory examinations revealed bicytopenia (hemoglobin 66 g/L, platelets 20×109/L, and leukocytes 11.2×109/L); hypofibrinogenemia (42 mg/dL, reference interval [RI]: 200–400 mg/dL); and elevated levels of ferritin (9,542.7 ng/mL, RI: 10–290 ng/mL), soluble CD25 (21,800 U/mL, RI: 122–496 U/mL), AST (565 IU/L, RI: 0–40 IU/L), ALT (283 IU/L, RI: 0–40 IU/L), and lactate dehydrogenase (600 IU/L, RI: 120–250 IU/L). Flow cytometry, which was performed as previously reported [2], revealed decreased intracellular perforin expression on the patient's natural killer (NK) cells compared with levels observed in a healthy individual (Fig. 1A). Flow cytometry [3] also revealed markedly reduced NK cell cytotoxic activity compared with activity observed in a healthy individual (Fig. 1B). A bone marrow examination, which was performed immediately after admission, showed active hemophagocytosis (Fig. 1C). Serologic tests were compatible with a remote infection of Epstein-Barr virus (EBV) or cytomegalovirus (CMV). Complete sequencing of the PRF1 gene revealed the following compound heterozygous mutations: c.65delC (p.Pro22Argfs*29) and c.1091T>G (p.Leu364Arg). Her father was heterozygous for the former mutation, and her mother was heterozygous for the latter (Fig. 2). The Leu364Arg substitution was considered to be a probably damaging variant (score: 0.978) by PolyPhen-2 analysis (http://genetics.bwh.harvard.edu/pph2/) and a disease-causing variant (probability: 0.895) by MutationTaster analysis (http://www.mutationtaster.org). Disease-associated variants were not detected in UNC13D. On the basis of these findings and established criteria, the patient was diagnosed as having HLH, specifically FHL2 [1]. After receiving treatment based on the HLH-2004 protocol, which included dexamethasone, etoposide, and cyclosporine A [4], the patient showed clinical and laboratory improvement. However, eight months later, the patient was found to have reactivation involving the central nervous system, which was treated by intrathecal methotrexate and repeated chemotherapy. Upon recovery, she underwent haploidentical hematopoietic cell transplantation (HCT) 12 months after her initial presentation. At the time of this publication, the patient had survived for 10 months post-transplantation, although she developed HCT-associated complications including reactivation of EBV and CMV infections.
The types of PRF1 mutations in Korean patients with FHL are notable for two characteristics. First, the frequency of PRF1 mutations was 4.2% among Korean patients in a recent study [5], whereas these mutations account for approximately 30–35% of FHL cases in other ethnic groups [1]. These low frequencies are accounted for by the unexpected predominance of UNC13D mutations (85% in a recent study) in Korean patients with FHL [5]. Second, a specific mutation, c.1090_1091delCT, is the most prevalent PRF1 mutation in Korean and Japanese patients, compared with that in other ethnic groups [56789]. Interestingly, this type of PRF1 mutation shares the same nucleotide region as the mutation observed in our patient (c.1091T>G). c.65delC is the only other mutation reported in Korean FHL2 patients [57]. Our patient had compound heterozygous mutations in the PRF1 gene. The c.1091T>G mutation has neither been previously reported as a single nucleotide polymorphism (SNP) nor as a mutation in the main genomic databases including the Human Gene Mutation Database, the SNP database (dbSNP), and ClinVar, whereas the c.65delC mutation was previously reported in Asian infants in a compound heterozygous manner [710].
In summary, we report a patient with typical HLH due to compound heterozygous mutations in PRF1, which manifested ascytotoxic lymphocyte dysfunction. To the best of our knowledge, this is the first case report worldwide of a patient with the PRF1 mutation c.1091T>G. This mutation shares the same site as the mutation c.1090_1091delCT, which is a unique PRF1 mutation in the Korean and Japanese populations.
References
1. Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013; 161:609–622. PMID: 23577835.
2. Kogawa K, Lee SM, Villanueva J, Marmer D, Sumegi J, Filipovich AH. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002; 99:61–66. PMID: 11756153.
3. Chung HJ, Park CJ, Lim JH, Jang S, Chi HS, Im HJ, et al. Establishment of a reference interval for natural killer cell activity through flow cytometry and its clinical application in the diagnosis of hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2010; 32:239–247. PMID: 19614711.
4. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007; 48:124–131. PMID: 16937360.
5. Seo JY, Song JS, Lee KO, Won HH, Kim JW, Kim SH, et al. Founder effects in two predominant intronic mutations of UNC13D, c.118-308C>T and c.754-1G>C underlie the unusual predominance of type 3 familial hemophagocytic lymphohistiocytosis (FHL3) in Korea. Ann Hematol. 2013; 92:357–364. PMID: 23180437.
6. Yoon HS, Kim HJ, Yoo KH, Sung KW, Koo HH, Kang HJ, et al. UNC13D is the predominant causative gene with recurrent splicing mutations in Korean patients with familial hemophagocytic lymphohistiocytosis. Haematologica. 2010; 95:622–626. PMID: 20015888.
7. Kim JY, Shin JH, Sung SI, Kim JK, Jung JM, Ahn SY, et al. A novel PRF1 gene mutation in a fatal neonate case with type 2 familial hemophagocytic lymphohistiocytosis. Korean J Pediatr. 2014; 57:50–53. PMID: 24578718.
8. Trizzino A, zur Stadt U, Ueda I, Risma K, Janka G, Ishii E, et al. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008; 45:15–21. PMID: 17873118.
9. Ishii E, Ohga S, Imashuku S, Kimura N, Ueda I, Morimoto A, et al. Review of hemophagocytic lymphohistiocytosis (HLH) in children with focus on Japanese experiences. Crit Rev Oncol Hematol. 2005; 53:209–223. PMID: 15718147.
10. Chiang GP, Li CK, Lee V, Cheng FW, Leung AW, Imashuku S, et al. Perforin gene mutation in familial haemophagocytic lymphohistiocytosis: the first reported case from Hong Kong. Hong Kong Med J. 2014; 20:339–342. PMID: 25104007.
Fig. 1
Results of intracellular flow cytometry, a cytotoxicity assay, and an assessment of bone marrow aspirate. (A) Intracellular perforin expression in naturl killer (NK) cells and cytotoxic T cells from the patient was reduced (0.4% and 11.5%, respectively), compared with a healthy control (68.8% and 13.4%, respectively, data not shown). (B) NK cell cytotoxicity was tested by incubating peripheral blood mononuclear cells with FITC-labeled NK-sensitive target cells (K562 cells). The cell population in upper right quadrant indicated killed K562 cells. NK cell cytotoxicity was impaired in the patient (3.2%, lower histogram) compared with a normal control (16.0%, upper histogram). (C) Bone marrow aspirate smear showed numerous histioytes engulfing various types of hematopoietic cells (Wright-Giemsa stain, ×1,000).
Abbreviations: SSC, side scattered light; FITC, fluorescein isothiocyanate; PI, propidium iodide.
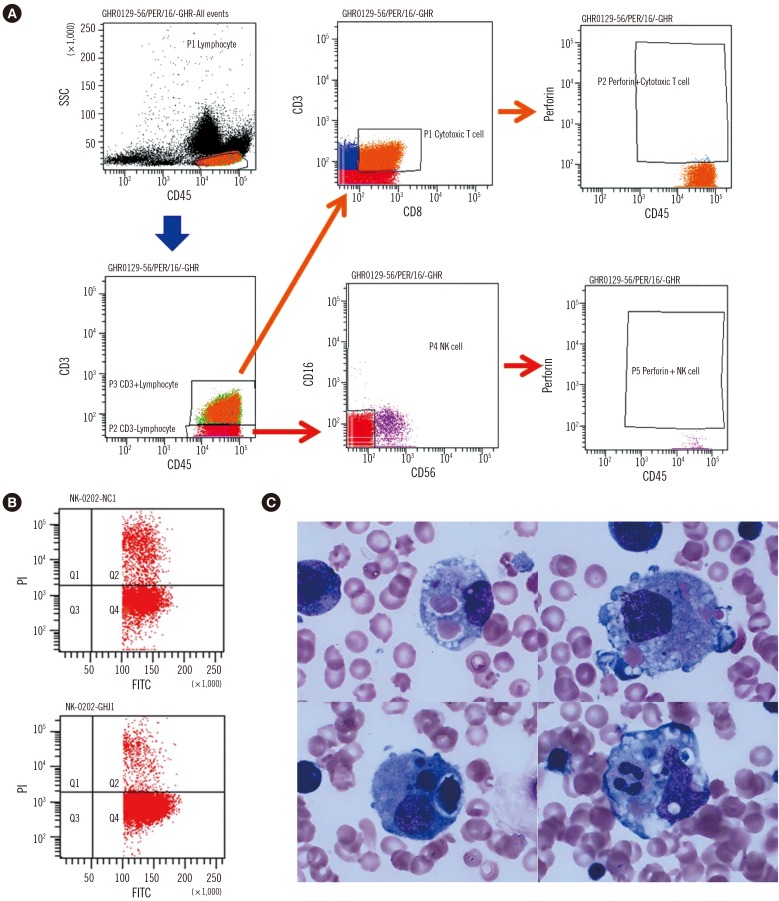
Fig. 2
Direct sequencing results of the PRF1 gene of the patient indicates compound heterozygous mutations. Upper panel shows an electropherogram of the c.65delC mutation (p.Pro22Argfs*29), which is the paternal allele. Lower panel shows an electropherogram of the c.1091T>G mutation (p.Leu364Arg), which is the maternal allele.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download