Abstract
Von Willebrand factor (vWF) is a glycoprotein with a crucial role in the formation of platelet thrombi, and ADAMTS13 is the main enzyme responsible for vWF cleavage. Both are important in the relationship between diabetic nephropathy, hypercoagulability, and cardiovascular disease. This study evaluated a potential relationship between vitamin D (vitD) levels, vWF, ADAMTS13 activity, and inflammation in diabetic patients on chronic hemodialysis (HD). Blood samples from 52 diabetic patients on chronic HD were obtained to determine vitD levels, vWF, and ADAMTS13 activity, and inflammatory markers. HD patients were grouped according to 25-hydroxyvitamin D [25(OH) VitD]<25 nmol/L (n=16) or >25 nmol/L (n=36). vWF antigen and vWF activity were elevated in both groups, with an average of 214.3±82.6% and 175.8±72.6%, respectively. Average ADAMTS13 activity was within the normal range in both groups. Blood samples from the vitD <25 nmol/L group showed a positive correlation between c-reactive protein (CRP) and vWF levels (P=0.023; r=0.564; 95% confidence interval=0.095-0.828), with a negative correlation between HbA1c and 25(OH) VitD (P=0.015; r=-0.337; 95% confidence interval=-0.337-0.19). Diabetic patients on chronic HD had elevated vWF levels and activity with no significant change in ADAMTS13 activity. The correlation between CRP and vWF levels in the 25(OH) VitD<25 nmol/L group suggests inflammatory-related endothelial dysfunction in these patients.
Diabetic kidney disease (DKD) is the most common cause of chronic kidney disease (CKD) worldwide, contributing to approximately 45% of new cases of end-stage renal disease [1]. Endothelial dysfunction and hypercoagulability are involved in the development of cardiovascular complications observed in patients with DKD [23]. von Willebrand factor (vWF), a multimeric glycoprotein synthesized and stored in endothelial cells and platelets, plays an important role in platelet thrombi formation. ADAMTS13 is a proteolytic enzyme expressed in endothelial cells and platelets that cleaves vWF [4]. Increased plasma levels of vWF might reflect damage to endothelial cells and a hypercoagulability state and have been associated with diabetes and atherosclerosis [5]. Recent studies report that, in patients with DKD, the observed high risk of cardiovascular complications might be associated with increased plasma levels of vWF and reduced ADAMTS13 levels [23]; these decreased ADAMTS13 levels might cause the increased vWF levels [235]. Thus, vWF and ADAMTS13 seem to be involved in the relationship between diabetes mellitus, kidney disease, hypercoagulability, and atherosclerotic cardiovascular diseases. Vitamin D (vitD) deficiency is associated with the development of diabetes mellitus and hypertension [67]. The current study investigated the relationship between vitD levels and plasma vWF and ADAMTS13 activity in diabetic patients on chronic hemodialysis (HD).
Fifty two diabetic patients on chronic HD for at least six months were enrolled from the Dialysis Unit at the Meir Medical Center, Israel. The medical records were reviewed to gather the data including age, sex, comorbidities, baseline laboratory data, and complications. There were 25 males and 27 females, with a mean age of 68±10 yr. Patients were grouped according to 25(OH) VitD levels: <25 nmol/L (n=16) or >25 nmol/L (n=36). Exclusion criteria were active malignancy, autoimmune disease, acute hospitalization, acute infection, and any unstable clinical conditions. Patients provided written informed consent to participate in the study, which was approved by the Meir Medical Center Ethics Committee. Blood samples were collected at the beginning of a dialysis session and sent to the laboratory.
Quantitative determination of vWF:Antigen (Ag) was performed by using a latex-enhanced immunoassay (Instrumentation Laboratory, Bedford, MA, USA). vWF:Activity was analyzed by using an INNOVANCE VWF Activity assay (Siemens Healthcare Diagnostics, Erlangen, Germany). Factor VIII activity was quantified on the basis of activated partial thromboplastin time (aPTT) assay using Factor VIII-deficient plasma (Instrumentation Laboratory).
ADAMTS13 activity levels were measured in plasma samples by a chromogenic enzyme-linked immunosorbent assay using a TECHNOZYM® ADAMTS13 Activity ELISA kit (Technoclone GmbH, Vienna, Austria). A monoclonal horseradish peroxidase (HRP)-conjugated antibody recognizes the cleaved substrate, and a chromogenic peroxidase reaction is used to determine the level of activated ADAMTS13 in the sample.
All data are expressed as mean±SD or median (range). Variables were tested for normality (Shapiro-Wilk test). For the variables which were not normally distributed, median and range were presented. Continuous parameters were compared with t-test or non-parametric Mann-Whitney test (each when appropriate). The comorbidities (nominal data) were compared to the 25(OH) VitD groups using Pearson Chi-Square test. Pearson product-moment correlation coefficient or Spearman Rho correlation was used to evaluate parametric data and non-parametric data, respectively, to measure linear correlations between blood measurement variables. P values <0.05 were considered significant.
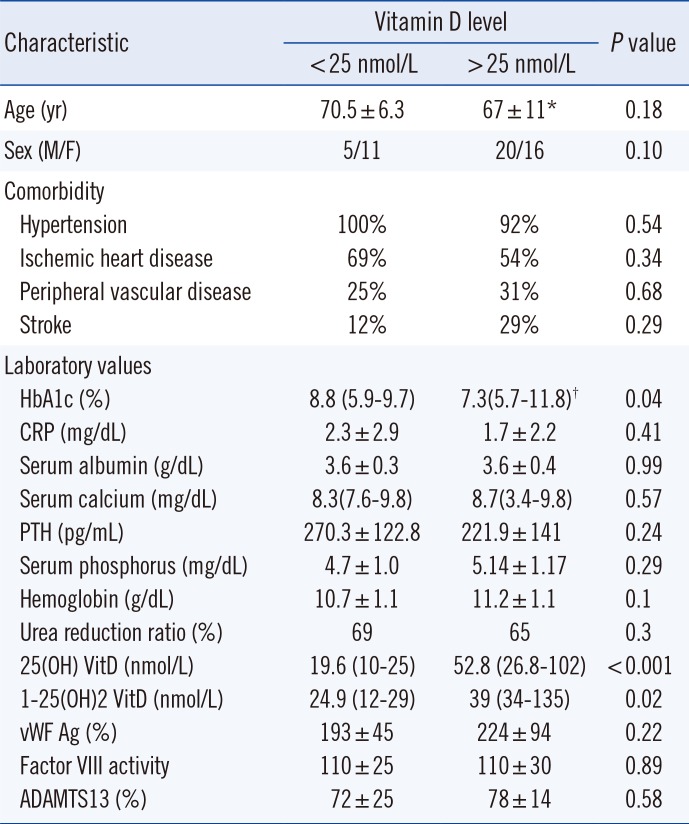
Most patients had comorbidities such as hypertension (94.2%), ischemic heart disease (55.8%), and peripheral vascular disease (28.8%). Patients were grouped according to 25(OH) VitD levels: <25 nmol/L (n=16) or >25 nmol/L (n=36) (Table 1). No significant differences were noted in most clinical and laboratory variables between the two groups. However, we observed significantly higher HbA1c levels in the 25(OH) VitD <25 nmol/L group than in the 25(OH) VitD >25 nmol/L group (median, range; 8.8%, 5.9-9.7% vs 7.3%, 5.7-11.8%, P=0.04). The average HbA1c level for the total study population was 7.6%; 61% participants had a level below 8%. We observed a negative correlation (P=0.015; r=-0.337; 95% [CI]=-0.337- 0.19) between HbA1c and 25(OH) VitD levels in the 25(OH) VitD <25 nmol/L group (P=0.015).
vWF Ag%, vWF activity, Factor VIII activity, and ADAMTS13 activity levels had normal distribution. vWF plasma levels were above reference values in both groups. The overall average was 214.3±82.6% (normal range 66-176%; Table 1). No significant differences in vWF were found according to vitD level. vWF activity was also elevated in the study population 175.8±72.6% (normal range 60-180%) with no significant differences between vitD groups (Table 1). Factor VIII activity in the overall study population was 111±28.4% (normal range 50-150%) with no significant differences between 25(OH) VitD groups (Table 1). ADAMTS13 activity levels analyzed in a subset of patients were within the normal range in both vitD groups 77.1±18.6%; (Table 1). We observed a trend toward higher inflammation levels (reflected by higher c-reactive protein [CRP] levels) in the 25(OH) VitD< 25 nmol/L group compared with the other group and a positive correlation between CRP and vWF levels (P=0.023; r=0.564; 95% CI=0.095-0.828), suggesting an inflammatory state and endothelial dysfunction (Fig. 1). No significant correlation was found between CRP and vWF levels in the 25(OH) VitD >25 nmol/L group (P=0.74; r=-0.058; 95% CI=-0.83-0.28).
Among 52 patients recruited to the study, eight died before the one-year follow-up. The leading cause of death was infection, followed by cardiovascular disease. Patients who died were older (76.9±7.9 vs 66.1±9.5 yr, P=0.004) than patients who survived until follow-up. One patient who died had lower ADAMTS13 activity (43.91%) compared with patients who survived until follow-up (80.71%±15.37%; P=0.05). Sixteen patients experienced non-fatal cardiovascular events. There was no significant correlation between the rate of cardiovascular events and 25(OH) VitD, vWF, or ADAMTS13 levels (data not shown). The study population experienced 122 hospitalizations. Among these, 41 were elective, related to vascular access, while 81 were due to other reasons. This confirms the well-documented high morbidity rate among diabetic patients on chronic HD. vWF activity and hospitalization rate were correlated (P=0.012; r=0.35; 95% CI=0.083-0.57).
Diabetic nephropathy is the leading cause of end-stage renal disease and is associated with high cardiovascular morbidity and mortality. In addition to conventional factors that contribute to vascular complications, hypercoagulability could play a role in cardiovascular disease among diabetic patients on chronic HD. Increased vWF levels and decreased ADAMTS13 activity have been reported in diabetic nephropathy and in patients on chronic HD [8]. An inflammatory state and advanced glycation end products (AGE) accumulation in patients on dialysis have been speculated to induce endothelial dysfunction and thereby increase vWF production [9]. The increased coagulability and inflammation observed in HD patients increase plasma levels of thrombin, plasmin, and granulocyte elastase, inducing ADAMTS13 degradation [10]. Therefore, the balance between decreased ADAMTS13 activity and increased vWF observed in inflammatory states and in dialysis patients may contribute to the prothrombotic state and accelerated atherosclerosis observed in this population [1112]. The association between diabetic nephropathy and increased cardiovascular morbidity and mortality is well-established, and platelet activation and hypercoagulability may contribute to the increased rate of cardiovascular events [1314]. Herein, we found increased levels of vWF without decreased ADAMTS13 activity. Increased vWF has been demonstrated in diabetic nephropathy, with a negative correlation between estimated glomerular filtration rate (eGFR) and vWF levels [411]. Lu et al [2] found increased plasma levels of vWF and reduced ADAMTS13 activity in patients with CKD compared with healthy subjects.
Few studies have evaluated plasma levels of vWF and ADAMTS13 activity in patients on chronic HD. Rios et al [15] found increased vWF levels and reduced ADAMTS13 activity without a significant correlation with the occurrence of vascular access thrombosis. We did not find decreased ADAMTS13 activity, similar to results obtained by Holden et al [16] who found increased levels of vWF antigen and activity, but no significant changes in ADAMTS13 activity in 55 patients on chronic HD. Absence of reduced ADAMTS13 activity observed in this study may be due to the use of different assays and possibility of an in vivo interaction preventing the cleavage of vWF multimers by ADAMTS13 [16]. De Filippis et al [17] demonstrated that vWF purified from dialysis patients was resistant to cleavage by ADAMTS13, possibly owing to high carbonyl levels at the cleavage site. Clinical settings of increased oxidative stress, including chronic HD, may inhibit degradation of vWF multimers by oxidative damage of MET 1606, which renders vWF resistant to proteolysis by ADAMTS13 [18].
All patients in this study had type 2 diabetes, which may have influenced the ADAMTS13 activity results. The trend toward increased mortality in patients with lower ADAMTS13 activity levels should be confirmed in larger prospective studies.
vWF level and ADAMTS13 activity were not affected by vitD. Nevertheless, we observed a significant correlation between CRP and vWF levels in the patients with 25(OH) VitD <25 nmol/L, suggesting that vitD deficiency can contribute to the inflammatory state and endothelial dysfunction in HD patients.
This study was limited by the relatively low number of subjects in each group, heterogeneity of the study population, and selection bias of a single center. The complexity of confounding factors in chronic HD patients requires larger prospective studies to confirm the possible association between vitD deficiency, increased vWF, reduced ADAMTS13, and cardiovascular outcomes.
Acknowledgments
We thank Dr. Eliezer Golan (Head of the Hemodialysis Unit) and Dr. Tali Tohami (Head of the Hematology Laboratory) at Meir Medical Center for their cooperation in performing this study; the medical editor, Faye Schreiber, for assistance in preparing the manuscript; and Nava Jelin, for assistance with statistical analysis.
References
1. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999; 100:1134–1146. PMID: 10477542.
2. Taniguchi S, Hashiguchi T, Ono T, Takenouchi K, Nakayama K, Kawano T, et al. Association between reduced ADAMTS13 and diabetic nephropathy. Thromb Res. 2010; 125:e310–e316. PMID: 20307901.
3. Domingueti CP, Dusse LM, Fóscolo RB, Reis JS, Annichino-Bizzacchi JM, Orsi FL, et al. Von Willebrand factor, ADAMTS13 and D-Dimer are correlated with different levels of nephropathy in type 1 diabetes mellitus. PLoS One. 2015; 10:e0132784. PMID: 26168189.
4. Lu GY, Shen L, Wang ZY, Guo XF, Bai X, Su J, et al. Significance of plasma von Willebrand factor level and von Willebrand factor-cleaving protease activity in patients with chronic renal diseases. Chin Med J. 2008; 121:133–136. PMID: 18272039.
5. Turner NA, Nolasco L, Ruggeri ZM, Moake JL. Endothelial cell ADAMTS-13 and VWF: production, release, and VWF string cleavage. Blood. 2009; 114:5102–5111. PMID: 19822897.
6. Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005; 28:1228–1230. PMID: 15855599.
7. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281. PMID: 17634462.
8. Cao WJ, Niiya M, Zheng XW, Shang DZ, Zheng XL. Inflammatory cytokines inhibit ADAMTS13 synthesis in hepatic stellate cells and endothelial cells. J Thromb Haemost. 2008; 6:1233–1235. PMID: 18433458.
9. Dugé de Bernonville T, Guyot S, Paulin JP, Gaucher M, Loufrani L, Henrion D, et al. Dihydrochalcones: Implication in resistance to oxidative stress and bioactivities against advanced glycation end-products and vasoconstriction. Phytochemistry. 2010; 71:443–452. PMID: 20022617.
10. Rios DR, Carvalho MG, Figueiredo RC, Ferreira CN, Rodrigues VL, Souza RA, et al. ADAMTS13 and Von Willebrand factor in patients undergoing hemodialysis. J Thromb Thrombolysis. 2012; 34:73–78. PMID: 22298244.
11. Kessler L, Wiesel ML, Attali P, Mossard JM, Cazenave JP, Pinget M. Von Willebrand factor in diabetic angiopathy. Diabetes Metab. 1998; 24:327–336. PMID: 9805643.
12. Dubin R, Cushman M, Folsom AR, Fried LF, Palmas W, Peralta CA, et al. Kidney function and multiple hemostatic markers: cross sectional associations in the multi-ethnic study of atherosclerosis. BMC Nephrol. 2011; 12:3. PMID: 21269477.
13. Taslipinar A, Yaman H, Yilmaz MI, Demirbas S, Saglam M, Taslipinar MY, et al. The relationship between inflammation, endothelial dysfunction and proteinuria in patients with diabetic nephropathy. Scand J Clin Lab Invest. 2011; 71:606–612. PMID: 21864054.
14. Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014; 124:2333–2340. PMID: 24892707.
15. Rios DR, Fernandes AP, Figueiredo RC, Guimarães DA, Ferreira CN, Simões E, et al. Relationship between ABO blood groups and von Willebrand factor, ADAMTS13 and factor VIII in patients undergoing hemodialysis. J Thromb Thrombolysis. 2012; 33:416–421. PMID: 22466813.
16. Holden RM, Tuttle A, Burbidge T, Hegadorn C, Grabell J, Pruss C, et al. Quantitative and qualitative changes of von Willebrand factor and their impact on mortality in patients with end-stage kidney disease. Blood Coagul Fibrinolysis. 2013; 24:719–726. PMID: 23846000.
17. De Filippis V, Lancellotti S, Maset F, Spolaore B, Pozzi N, Gambaro G, et al. Oxidation of Met1606 in von Willebrand factor is a risk factor for thrombotic and septic complications in chronic renal failure. Biochem J. 2012; 442:423–432. PMID: 22091998.
18. Lancellotti S, De Filippis V, Pozzi N, Peyvandi F, Palla R, Rocca B, et al. Formation of methionine sulfoxide by peroxynitrite at position 1606 of von Willebrand factor inhibits its cleavage by ADAMTS-13: A new prothrombotic mechanism in diseases associated with oxidative stress. Free Radic Biol Med. 2010; 48:446–456. PMID: 19969076.
Fig. 1
Positive correlation between CRP and vWF in hemodialysis patients with 25(OH) VitD <25 nmol/L (P=0.023; r=0.564). Regression line: y=7.52x+175.88
Abbreviations: CRP, c- reactive protein; vWF, von Willebrand factor; Ag, antigen.
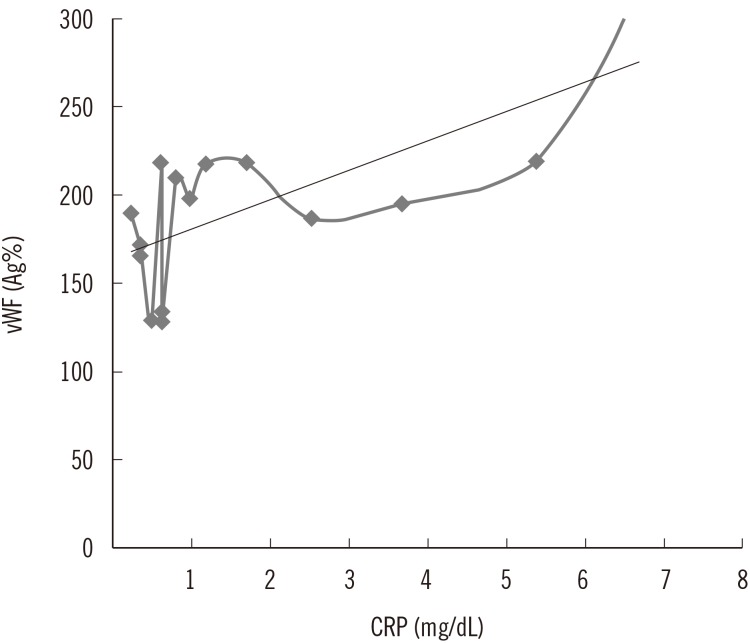
Table 1
Clinical characteristics of diabetic HD patients according to 25(OH) vitamin D levels




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download