Abstract
From 2013 to 2015, the National Institute of Health, Pakistan, received 1,270 blood samples of suspected dengue cases reported from inpatient and outpatient departments of various hospitals in Khyber Pakhtunkhwa (KPK) province. In this study, we determined the circulating dengue virus (DENV) serotypes using real-time reverse transcriptase (RT)-PCR to understand the serotype-based epidemiology of DENV. All four serotypes (DENV-1 [6%], DENV-2 [33%], DENV-3 [47%], and DENV-4 [0.1%]) were found circulating during the study period. Our findings suggest the need for an active surveillance system coupled with the laboratory diagnosis, especially in the chronic endemic areas of the country. Public awareness programs are needed for effective control and prevention of outbreaks in the future.
Dengue fever is an important emerging and re-emerging arboviral infection and represents a major public health problem in tropical and subtropical regions of the world [1]. Dengue fever is caused by dengue virus (DENV), which belongs to the flavivirus genus within the family flaviviridae [2]. DENV has been classified into four serotypes (DENV-1-4) [3].
Dengue is a known vector-borne disease in many Southeast Asian countries, including India and Pakistan [45]. The DENV is transmitted by mosquitos, mainly Aedes aegypti and Aedes albopictus. DENV infection is classified into three categories ranging from mild dengue fever to severe life threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [6].
Estimates provided by the World Health Organization indicate that 2.5 billion people among 124 countries are at risk of dengue infection, with over 100 million cases of DENV infection and 30,000 estimated deaths from infection reported globally each year. There are approximately 500,000 annual cases of DHF and DSS, which are related to severe disease manifestations due to secondary dengue infections [7]. The annual incidence rates of dengue cases in Pakistan for the years 2013, 2014, and 2015 were 0.4/1,000,000, 0.1/1,000,000, and 0.5/1,000,000, respectively.
Pakistan experiences a number of dengue outbreaks every year in urban regions, mainly in the Sindh and Punjab provinces. In the last few years, there has been an increasing trend in the number of dengue cases reported from Khyber Pakhtunkhwa (KPK) province [1]. KPK province is one of the four provinces of Pakistan; it is located in the north-western region of the country with an area of 74,521 km2 and a population of 26.9 million.
From August 2013 to November 2015, a total of 1,270 blood samples collected from suspected dengue cases were received from KPK province and processed at the Department of Virology, National Institute of Health (NIH), Islamabad for laboratory confirmation of dengue. These samples were collected from hospitals in four major districts of KPK province (i.e., Swat, Mansehra, Malakand, and Kohat). The study concept and design were approved by the Internal Review Board of NIH, Islamabad.
Viral RNA was extracted from 140 µL serum samples by using a Qiamp Viral RNA extraction kit (Qiagen GmBH, Hilden, Germany) according to the manufacturer's protocol with the exception of elution volume, which was 60 µL and stored at -70℃ until further analysis. A one-step real-time TaqMan reverse transcriptase (RT)-PCR for detecting and typing DENVs was carried out according to Barbara et al [8]. Briefly, singleplex reaction mixture of 25 µL was run for each DENV serotype (DENV-1-4) in an ABI7500 real-time thermocycler by using the SuperScript III Platinum one-step qualitative RT (qRT)-PCR kit (Invitrogen, Carlsbad, CA, USA). Amplifications for each serotype were carried out in a 25-µL reaction mixture containing 5 µL RNA, 12.5 µL of 2X reaction mixture, 0.5 µL of enzyme mix, 2 µM of each primer, and 1 µM of TaqMan probe. The cycling conditions were as follows: RT step 50℃ at 10 min, initial denaturation at 95℃ for 5 min, 45 cycles at 95℃ for 15 sec, and final extension at 60℃ for 60 sec. The data were analyzed by using software SDS version 1.4 (Applied Biosystem, Foster City, CA, USA).
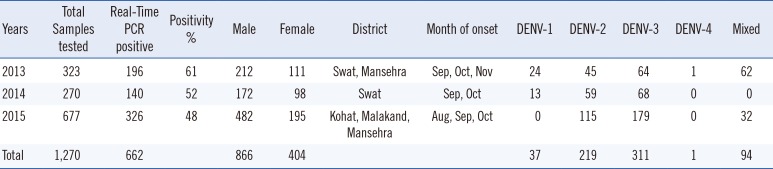
Of the total 1,270 samples, 68% (866/1,270) were collected from male and 32% (404/1,270) from female patients; the male to female ratio was 2:1. Out of the 1,270 samples, 52% (662/1,270) were positive for dengue infection, with 61% (196/323), 52% (140/270), and 48% (326/677) positive in 2013, 2014, and 2015, respectively. During 2013-2015, DENV-3 was the most dominant serotype (47%; 311/662 cases), followed by DENV-2 (33%; 219/662 cases). DENV-1 was detected in 6% (37/662) of samples, and only one sample tested positive for DENV-4 in 2013. Presence of DENV-4 RNA in one sample was also confirmed by retesting and by sequencing the E-NS1 gene. Blast and phylogenetic analyses of the sequence derived from this sample showed that the DENV sequence belonged to DENV-4 serotype and genotype I, closely related to DENV-4 detected from India and Pakistan.
The majority of dengue cases in 2013 were from Swat (71%). In 2014, all 270 cases were from Swat; in 2015, (81%) were from Mansehra, (15%) from Kohat, and only (4%) from the Malakand district. Young adults (ages 16-30 yr) were the most affected age group during the 2013-2015 outbreaks. The seasonal trend of dengue cases was reflected by the peak of positive cases detected during post-monsoon season during all three study years (Table 1).
The highest positivity rate was shown for the samples collected within 4-6 days after onset of symptoms (day 4, 55%; day 5, 66%; day 6, 55%). The positivity rate dropped to almost zero for samples collected beyond 11 days after the onset of symptoms (Fig. 1).
Dengue is endemic in Pakistan, and the first outbreak was reported in 1994 in Karachi city, Sindh Province. The two largest dengue outbreaks were reported for the first time in the country during 2011 in Lahore and in 2013 in Swat. During 2011, Lahore faced a burden of 22,562 cases and 363 deaths, and Swat in 2013 reported 8,343 dengue cases and 57 deaths. Predominant circulating DENV serotypes reported were DENV-2, DENV-3, and DENV-4 from Lahore in 2011, and DENV-2 and DENV-3 from Swat in 2013 [179]. These findings led us to study the current epidemiological patterns of dengue infection and understand the prevailing DENV serotypes in the country's endemic areas.
Various diagnostic methods are currently available for the diagnosis of DENV infection. Virus isolation, conventional RT-PCR, real-time PCR, genomic sequencing, and ELISA are used for detection of DENV [10]. In this study, we used real-time RT-PCR, which is among the most sensitive molecular techniques with the advantage of being able to detect both DENV RNA and serotype simultaneously.
Of the 1,270 samples analyzed, 52% (662/1,270) were positive for dengue infection, which probably reflects timely sample collection after the onset of symptoms. The suitable period for the detection of viral RNA is usually 2-7 days after onset of infection. After day 7, IgM antibodies appear in the blood and viral load decreases. The sample for viral antigen or RNA detection must be collected during the viremic phase when the viral load in the blood is high; however, the majority of suspected dengue patients often arrive at the health facility several days after the onset of infection [11].
In 2013, KPK province was hit by a major dengue outbreak infecting more than 11,000 individuals, mainly in the Swat district, and lasting until 2014. During 2015, dengue cases continued to be reported from the neighboring areas, including the Malakand, Kohat, and Mansehra districts, with sporadic cases reported from districts of Upper and Lower Dir and Shangla, which are in close proximity to Swat [1].
During 2013 to 2015, individuals aged 16-30 yr of age were the main victims of DENV infection, followed by individuals between 31-45 yr of age [112]. Demographic data analysis showed that the infection rate in males was double the rate in females, with a male to female ratio of 2:1, an observation that corresponds with previous results from studies of the KPK province [1213].
According to the results presented here, we confirmed DENV infection due to all four serotypes; in contrast, previous reports using data collected during the same time period and from the same geographical areas only confirmed DENV-2 and DENV-3 in samples [14]. In this study, we found DENV-1 in 6% (37/662) of samples and DENV-4 was detected for the first time from KPK Province during this study, which indicates the silent circulation of DENV-4 in this area. This result may be attributed to the use of highly sensitive real-time RT-PCR in our study compared with the conventional RT-PCR used in previous studies [14]. The highest number of cases in our study were detected from the time period during the post-monsoon period, which might be due to increased humidity and precipitation, the most suitable environment for vector breeding. This observation is consistent with reports of dengue outbreaks in other provinces, as well as from various studies conducted globally [151617].
Our study emphasizes that in countries with known endemicity of DENV disease, standard diagnostic measures must be introduced and implemented in order to obtain accurate information on the circulating DENV serotypes. It is known that secondary infections occurring in the DENV endemic regions or areas with co-circulation of multiple DENV serotypes represents a major public health threat and indication of progress towards DHF and DSS [1819].
In conclusion, the present data enhance our existing knowledge about the circulating DENV serotypes in the KPK province of Pakistan. An active disease surveillance system must be introduced in the country, along with concomitant public awareness programs, for effective prevention and control of DENV infections in Pakistan.
References
1. World Health Organization. Dengue and severe dengue. Fact sheet No. 117. 2015. Updated on Jul 2016. http://www.who.int/mediacentre/factsheets/fs117/en/.
2. Guzmán MG, Kourí G. Dengue: an update. Lancet Infect Dis. 2002; 2:33–42. PMID: 11892494.
3. Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011; 29:587–619. PMID: 21219187.
4. Halstead SB. Dengue. Lancet. 2007; 370:1644–1652. PMID: 17993365.
5. WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. 2009 New edition. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf.
6. Gubler DJ. The global pandemic of dengue/dengue haemorrhagic fever: current status and prospects for the future. Ann Acad Med Singapore. 1998; 27:227–234. PMID: 9663316.
7. WHO. WHO Weekly Epidemiological Monitor. Vol. 6, Issue 52, 29 December, 2013. Dengue in Pakistan. http://reliefweb.int/sites/reliefweb.int/files/resources/Epi_Monitor_2013_6_52.pdf.
8. Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005; 43:4977–4983. PMID: 16207951.
9. Ali A, Ahmad H, Idrees M, Zahir F, Ali I. Circulating serotypes of dengue virus and their incursion into non-endemic areas of Pakistan; a serious threat. Virol J. 2016; 13:144. PMID: 27565893.
10. De Paula SO, Fonseca BA. Dengue: a review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz J Infect Dis. 2004; 8:390–398. PMID: 15880229.
11. Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infection. J Infect Dis. 2002; 185:1213–1221. PMID: 12001037.
12. Ali A, Rehman HU, Nisar M, Rafique S, Ali S, Hussain A, et al. Seroepidemiology of dengue fever in Khyber Pakhtunkhawa, Pakistan. Int J Infect Dis. 2013; 17:e518–e523. PMID: 23523057.
13. A joint report of World Health Organization and Health Department, Government of Khyber Pakhtunkhwa. Swat: Dengue fever snapshot (07 August-20 October 2013). http://reliefweb.int/sites/reliefweb.int/files/resources/Swat_dengue_fever_snapshot_211013.pdf.
14. Ali A, Nasim Z, Rehman RU, Farzana AS, Zahir F, Iqbal A, et al. Dengue virus serotype 2 and 3 causing high morbidity and mortality in Swat, Pakistan. Biohelikon Immun Dis. 2013; 1:1–3.
15. Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J. 2006; 3:92. PMID: 17083743.
16. Akhtar N, Khan J, Khan A. Dengue outbreak in Khyber Pakhtoonkhwa, Pakistan 2013. Eur Acad Res. 2014; 1:3842–3857.
17. Pandey BD, Morita K, Khanal SR, Takasaki T, Miyazaki I, Ogawa T, et al. Dengue virus, Nepal. Emerg Infect Dis. 2008; 14:514–515. PMID: 18325280.
18. Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988; 239:476–481. PMID: 3277268.
19. Huhtamo E, Hasu E, Uzcátegui NY, Erra E, Nikkari S, Kantele A, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol. 2010; 47:49–53. PMID: 19963435.
Fig. 1
Results of real-time reverse transcriptase (RT)-PCR according to the days after onset of symptoms.
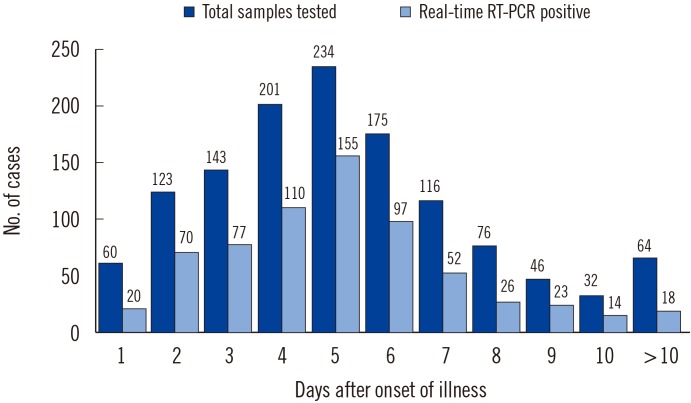
Table 1
Demographic and molecular characteristics of dengue cases in Khyber Pakhtunkhwa (KPK) province, Pakistan, 2013-2015




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download