INTRODUCTION
Umbilical cord blood is recognized as a source of allogeneic hematopoietic stem cells since the first successful cord blood transplantation in a patient with Fanconi anemia [
1]. Hundreds of thousands of processed cord bloods units (CBUs) are stored globally, and maintaining the quality of long-term cryopreserved cord blood has become an important issue. External and internal quality control programs for cryopreserved cord blood are implemented in each cord blood bank. Total nucleated cell (TNC) count, CD34
+ cell count, cell viability, and colony-forming units (CFU) are the commonly analyzed quality control variables used by both external and internal programs.
While external quality control programs are usually operated by government-related organizations or government-accredited institutions (i.e., EUROCORD, NMDP), internal quality control programs are conducted by the cord blood bank itself at each processing step. According to the Korean law, internal quality control analysis for maintaining the quality of cryopreserved cord blood should be performed monthly, but specific details besides laboratory tests are not described. In our previous study, we evaluated the quality of 200 CBUs that were cryopreserved for 1–5 yr and concluded that cryopreservation does not affect the quality of CBUs [
2]. In line with quality maintenance for a defined period, we describe the results of the internal quality control program thus far, to see the feasibility of our long-term internal quality control program for the cord blood bank, which will continue over a 21-yr period.
Go to :

METHODS
1. Processing and measurement of cord blood variables
CBUs were collected after obtaining informed consent from the mother and were processed for cryopreservation according to the standard operating procedures of Seoul Metropolitan Government Public Cord Blood Bank (Allcord) [
34]. The collection bag weight was measured, and the blood volume was calculated with the density factor of 1.05 g/mL. Cord blood samples with a volume >60 mL were then assessed for complete blood count and cell viability (pre-processing viability).
Nucleated cell concentrates (20 mL) were obtained after removing red blood cells and extra plasma from cord blood using a transfer/freezing bag set (Pall Medical, Covina, CA, USA). The nucleated cell concentrate was then assessed for the TNC and CD34
+ cell counts. The TNCs were determined by using an automated cell counter (XE-2100; Sysmex, Kobe, Japan). CD34
+ cell determination was conducted as previously described by using a Stem-kit (#IM3630, BD Bioscience, San Jose, CA, USA) [
2], on the basis of the single-platform International Society of Hematotherapy and Graft Engineering method. After adding 5 mL of cryoprotectant (2.5 mL of dimethyl sulfoxide [Mylan, Rockford, IL, USA] and 2.5 mL of 10% dextran [Daihan, Namyangju, Korea]), the nucleated cell concentrate resulted in a final volume of 25 mL for each CBU. Pre-cryopreservation cell viability was tested by staining with 0.4% trypan blue, and viabilities over 80% were considered acceptable. The CBUs were transferred to a controlled-rate freezer (CryoMed, Thermo Forma, Marietta, OH, USA) for cryopreservation.
2. Sample selection for long-term internal quality control plan
Once a month, donated cord blood that was unsuitable for storage as a CBU for allogeneic transplantation [
5] was selected. The selected nonconforming CBU in this study was a sample with an initial TNC level <8×10
8/CBU that had no other reason for unsuitability. This study using the nonconforming CBUs as quality control materials was categorized as an exempt review by the Institutional Review Board of Boramae Hospital (07-2016-1).
3. Preparation of quality control materials
The selected nonconforming CBU was processed with the same steps used for conforming CBUs. The nonconforming CBU ready for cryopreservation was aliquoted into 21 capillaries, which would be cryopreserved for the next 21 yr. Using a 10 mL syringe, 0.3–0.4 mL of cord blood was filled into each of the 21 capillaries (Medium straws #005569, IMV Technologies, L'Aigle, France), which were then sealed at both ends (
Fig. 1). Each capillary was placed into an overwrap bag, and placed in canisters (3 capillaries/canister); there were seven canisters for each CBU, which were stored in liquid nitrogen for future use.
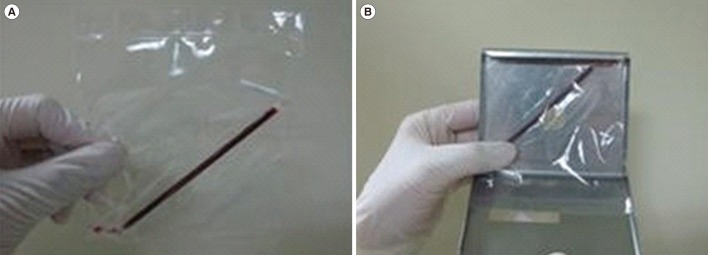 | Fig. 1Capillary preparation for the quality control program. (A) An overwrapped capillary containing 300–400 µL of cord blood sealed at both ends. (B) An overwrapped capillary placed into a canister, which was transferred to a liquid nitrogen tank, similar to the storage conditions used for cord blood units.
|
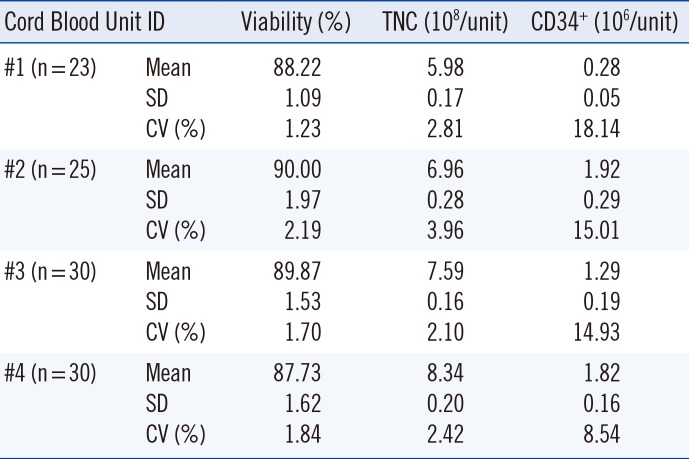
4. Evaluating homogeneity of capillaries from the same CBU
In order to pre-evaluate the reproducibility of the cord blood variables for each capillary, 4 CBUs in the pre-cryopreservation state were selected and aliquoted into 23–30 capillaries. Capillaries were stored in liquid nitrogen and thawed seven days later. Cell viability (post-thawing), TNC count, and CD34+ cell count were analyzed for each capillary, and the reproducibility of the measurements among capillaries from each CBU was assessed.
5. Assessment of cord blood variables in capillaries cryopreserved for one year
Cord blood capillaries that were to be tested each month were removed from the freezer and thawed in a 37℃ water bath; a similar procedure was used when thawing the segment attached to the main bag for a transplantation verification test. After thawing, the cord blood was poured into a test tube by incising the ends of the capillaries, and the cord blood variables (TNC and CD34
+ cell count, cell viability test, and CFU assay) were assessed using previously described methods [
23]. In this study, the first capillary was thawed and analyzed after one year of storage. Afterwards, one capillary from each CBU will be analyzed every year.
6. The quality control samples for the pilot study
The first CBU capillaries were prepared in October 2012, with one new set prepared each month since. The samples for the pilot study included the samples from October 2012 to January 2014. The 3 CBUs that were processed in 2012 plus the 12 CBUs processed every year from 2013 to 2032 will add up to a total of 243 CBUs. Homogeneity of the capillaries was represented as a CV of 7-day-frozen and thawed capillaries. The blood variables were compared for the first and second year of storage to investigate the effects of cryopreservation duration.
7. Statistical analysis
A Wilcoxon signed-rank test was performed to determine the statistical significance of the variable changes between before freezing and after thawing, and also according to the duration of cryopreservation. P values < 0.05 were considered significant (IBM SPSS Statistics 24, Chicago, IL, USA). The variables of cryopreserved cord blood were presented as median and the range in parentheses.
Go to :

DISCUSSION
Previous studies suggested that variables of cord blood quality correlate with the engraftment success rate and long-term survival of the patient [
67]. A cord blood Apgar score has been recently developed to measure the engraftment potential of cord blood [
8]. Maintaining cord blood quality during cryopreservation has become an important issue because umbilical cord blood is more readily available than ever before.
In this study, we present the result of a pilot study as a part of a 21-yr plan for the internal quality control program in our cord blood bank. Besides the relative long-term period of evaluation comparable to other studies [
910], our study has three advantages over previous studies assessing CBU quality [
910111213]. First, while many previous studies compared groups of CBUs that had been stored for a specific duration, we planned to aliquot a single unit into 21 capillaries that can be tested over time. Therefore, we can compare multiple results (a maximum of 21 results) from one CBU over 21 yr if we test a capillary once per year. Second, the containers for evaluating cryopreserved CBU have typically been cryotubes [
10], which are generally used in pre-clinical research laboratories, but we introduced capillaries that are suitable for long-term preservation of live reproductive cells. Third, the capillaries were cryopreserved in liquid nitrogen to simulate the environment used for storing conforming CBUs. Furthermore, the capillary preparation procedure was simple and allowed easy, long-term follow-up of the same CBU. The CV among capillaries of each CBU showed high reproducibility for viability and TNC counts. The CD34
+ cell counts showed higher CV% than other variables, which may be due to the relatively low levels of CD34
+ cell counts in the samples used to validate reproducibility. This capillary method can be used for preparing materials for inter-laboratory external proficiency tests, because the capillaries originate from the same CBU.
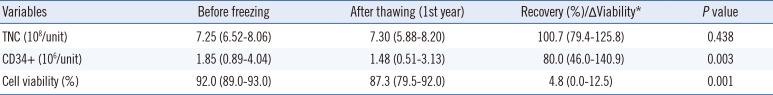
According to our data, thawing the cryopreserved CBUs resulted in a significant decrease in the CD34
+ cell counts and cell viabilities. This decrease in CD34
+ cells and cell viability were similar to the rates reported previously [
11]. Although some reports demonstrated an overall decrease in TNCs of approximately 11–30% [
111213], we observed stable TNC levels during upon thawing, and this observation is in line with our previous results [
2]. Since the sample numbers, preservation condition (vapor or liquid phase of liquid nitrogen), preservation periods, and the time of sampling (immediate post-thaw or after-washing state) for each study were too diverse to reach a robust conclusion regarding any changes in TNC, we expect that further studies will determine if cryopreservation period affects the TNC.
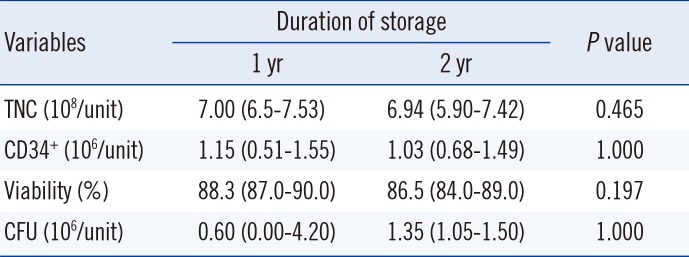
On the other hand, the difference in the cryopreservation period did not result in any significant changes in TNC, viability, or CD34
+ cell count between the capillaries stored for one or two years. In previous studies, a significant increase in the percentage of cells expressing CD34
+ was observed with increasing storage time [
1214]. These studies involved CBUs from multiple institutions, which indicates the challenge in quantifying CD34
+ cells. When multiple cryopreserved capillaries from the same CBUs are available for internal quality control analysis over 21 yr, differences or deviations in the sample test results may be detected more thoroughly.
This study has some limitations. First, we were only able to conduct a short-term analysis of our long-term plan using the pilot data. Second, this study was based on only in vitro tests, which focused on the quality control of stored CBUs, and did not reflect the clinical outcome of transplanted cord blood according to the variables analyzed.
In summary, we proposed a long-term internal quality control plan for cord blood banks, which can be used to assess the markers used for quality assessment during long-term cord blood storage. Using cord blood in capillaries, which can be easily stored for a long period, is similar to testing integral segments attached to each CBU. In this pilot study, consisting of 16 and four cord blood samples that were analyzed in the first and second year of storage, respectively, we observed no meaningful changes in CBU quality during cryopreservation. To the best of our knowledge, this is the first study to suggest a detailed plan for a cord blood bank internal quality control program in Korea. We hope that this will lead to the standardization of internal quality programs, which can improve the reliability of CBU cryopreservation. Although most CBUs used for transplantation are only stored for three to five years, the results of this study could be useful for determining the shelf life of cryopreserved CBUs for transplantation.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download