Abstract
Background
Methods
Results
References
Table 1
Results of ABO blood typing from the AutoVue system
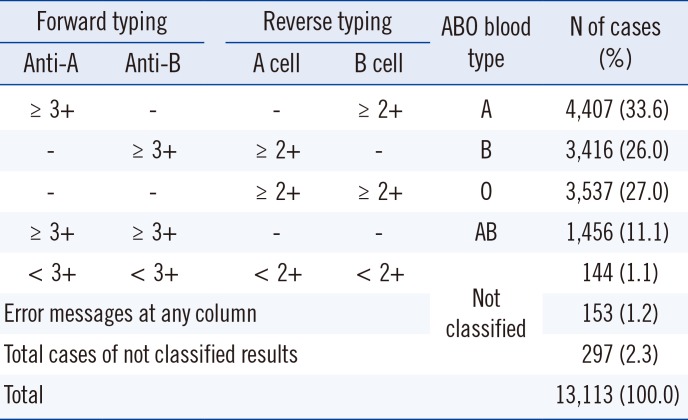
Forward typing, ABO blood typing with antibody reagent; Reverse typing, ABO blood typing with red cell; A cell, A1 phenotype red cell reagent; B cell, B phenotype red cell reagent; ≥3+, ≥3+ reaction grade; ≥2+, ≥2+ reaction grade; <3+, <3+ reaction grade; <2+, <2+ reaction grade; -, no reaction; Error messages at columns, the AutoVue system's specific error messages are presented at any column of the cassette (the cassette are composed of anti-A, anti-B, anti-D, A cell and B cell column); Not classified, the samples with discrepancies or error messages at any column of the cassette are not classified into ABO blood group.
Abbreviations: Anti-A, anti-A reagent; Anti-B, anti-B reagent.
Table 2
Results of ABO blood typing from the manual method
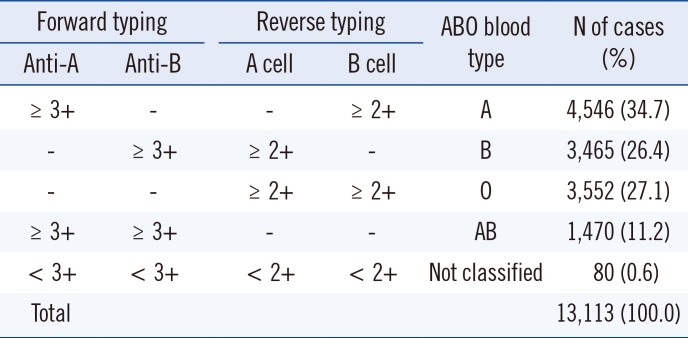
Forward typing, ABO blood typing with antibody reagent; Reverse typing, ABO blood typing with red cell; A cell, A1 phenotype red cell reagent; B cell, B phenotype red cell reagent; ≥3+, ≥3+ reaction grade; ≥2+, ≥2+ reaction grade; <3+, <3+ reaction grade; <2+, <2+ reaction grade; -, no reaction; Error messages at columns, the AutoVue system's specific error messages are presented at any column of the cassette (the cassette are composed of anti-A, anti-B, anti-D, A cell and B cell column); Not classified, the samples with discrepancies at any column of the cassette are not classified into ABO blood group; numbers in parentheses indicate the % of cases among the total cases.
Abbreviations: Anti-A, anti-A reagent; Anti-B, anti-B reagent.
Table 3
The discrepant results of ABO blood typing from the AutoVue system and manual method
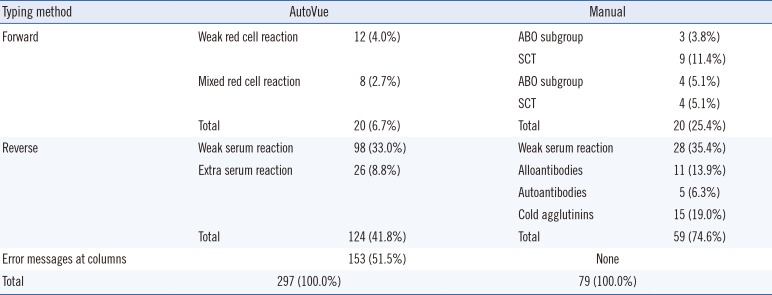
Typing method, ABO blood typing method; Forward, Forward typing; Reverse, Reverse typing; Error messages at columns, the AutoVue system's specific error messages are presented at any column of the cassette (the cassette are composed of anti-A, anti-B, anti-D, A cell and B cell column); Results, discrepant results; Weak red cell reaction, samples with <3+ reaction grade; Mixed red cell reaction, samples with mixed field reaction; Weak serum reaction, samples with <2+ reaction grade; Extra serum reaction, samples with unpredicted extra reaction at reverwse typing; AutoVue, the AutoVue system; Manual, the manual method; SCT, samples of the patients who had a hematopoietic stem cell transplant; ABO subgroup, samples defined as ABO blood subgroup by additional methods; Alloantibodies, samples with alloantibodies in serum; Autoantibodies, samples with autoantibodies in serum; Cold agglutinins, samples with cold agglutinins in serum; Numbers in parentheses indicate the % of cases showing discrepant results among the total discrepant cases respectively.
Abbreviation: SCT, stem cell transplanted.
Table 4
Results showing a weak serum reaction from the AutoVue system
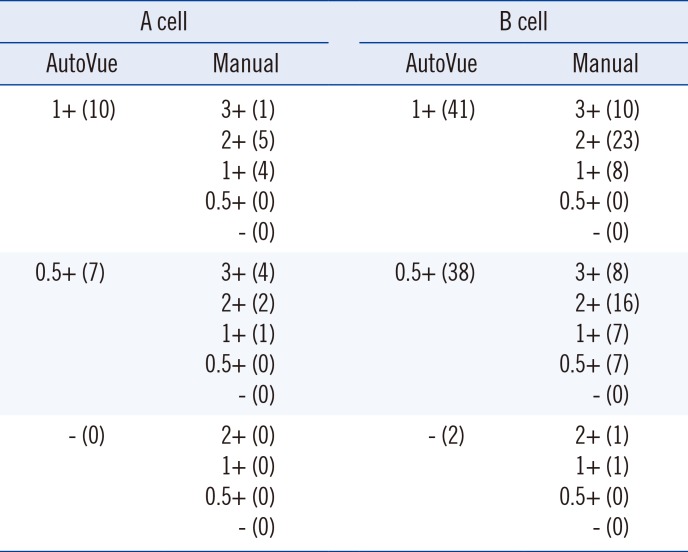
AutoVue, the AutoVue system; Manual, the reference manual method; A cells, A1 phenotype red cell reagent; B cell, B phenotype red cell reagent; 3+, 3+ reaction grade; 2+, 2+ reaction grade; 1+, 1+ reaction grade; 0.5+, 0.5+ reaction grade; -, no reaction; numbers in parentheses indicate the number of cases showing a specific reaction grade.
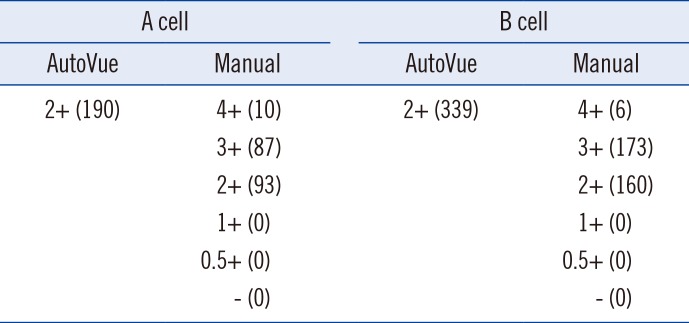
Table 5
Results showing a 2+ serum reaction grade in the AutoVue
| A cell | B cell | ||
|---|---|---|---|
| AutoVue | Manual | AutoVue | Manual |
| 2+ (190) | 4+ (10) | 2+ (339) | 4+ (6) |
| 3+ (87) | 3+ (173) | ||
| 2+ (93) | 2+ (160) | ||
| 1+ (0) | 1+ (0) | ||
| 0.5+ (0) | 0.5+ (0) | ||
| - (0) | - (0) | ||
AutoVue, the AutoVue system; Manual, the reference manual method; A cells, A1 phenotype red cell reagent; B cell, B phenotype red cell reagent; 4+, 4+ reaction grade; 3+, 3+ reaction grade; 2+, 2+ reaction grade; 1+, 1+ reaction grade; 0.5+, 0.5+ reaction grade; -, no reaction; numbers in parentheses indicate the number of cases showing a specific reaction grade.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download