This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Bone marrow chimerism (BMC) develops after allogeneic hematopoietic stem cell transplantion (HSCT); however, BMC is also associated with graft-versus-host disease (GVHD) following liver transplantation, which has an incidence rate of 0.1-2% and a mortality rate exceeding 75% [
1]. Therefore, detecting BMC is also important in solid organ transplantation (SOT) as well as HSCT, and accurate quantification is needed, especially in case of clonality distinction of minimal portions. This study aimed to introduce the significance of BMC in SOT, by using conventional short tandem repeat (STR)-PCR [
234] and next generation sequencing (NGS), through relative quantification of single-nucleotide polymorphisms (SNP).
A 55-yr-old Korean woman underwent liver transplantation owing to decompensated hepatitis C virus-related cirrhosis. The donor was a 40-yr-old woman who died from subarachnoid hemorrhage due to Moyamoya disease. After the operation, the patient received immunosuppressive therapy consisting of prednisone, one injection of basiliximab, and low-dose tacrolimus, which was discontinued when GVHD symptoms appeared. The patient developed fever of 39℃ 23 days after the transplant, with five days of severe leucopenia (0.38×109 white blood cells [WBC]/L), accompanied by anemia (6.4g of Hb/dL) and thrombocytopenia (81×109 platelets/L) with symptoms suggesting GVHD. Pancytopenia persisted despite administration of granulocyte-colony-stimulating factor. Bone marrow was aspirated to evaluate the etiology of hematologic disorder and to diagnose GVHD. Hypocellular marrow was observed, and BMC was analyzed simultaneously. The therapy was initiated to control GVHD, including substitution of tacrolimus with cyclosporin A and increasing mycophenolate mofetil. However, despite transfusion support, broad-spectrum antibiotics, and antifungal therapy, the patient died of sepsis due to Klebsiella pneumoniae infection.
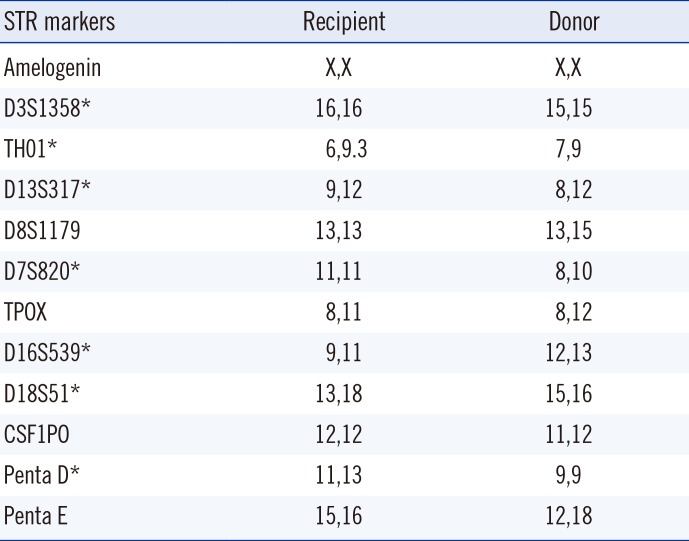
BMC was analyzed with stored, pre-transplant WBC pellets from both recipient and donor. DNA was extracted from the pellets with QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Chimerism was analyzed by STR-PCR using the GenomeLab Human STR Primer Set (Beckman Coulter, Fullerton, CA, USA), in which 11 polymorphic STR alleles were amplified and labeled with different fluorescent dyes. The PCR products were separated in a CEQ 8800 Capillary Electrophoresis System (Beckman Coulter). The percent chimerism was estimated as the average of percent recipient peaks observed for each distinguishable marker [
5] (
Table 1).
Table 1
Recipient pre-transplant and donor STR alleles
|
STR markers |
Recipient |
Donor |
|
Amelogenin |
X,X |
X,X |
|
D3S1358*
|
16,16 |
15,15 |
|
TH01*
|
6,9.3 |
7,9 |
|
D13S317*
|
9,12 |
8,12 |
|
D8S1179 |
13,13 |
13,15 |
|
D7S820*
|
11,11 |
8,10 |
|
TPOX |
8,11 |
8,12 |
|
D16S539*
|
9,11 |
12,13 |
|
D18S51*
|
13,18 |
15,16 |
|
CSF1PO |
12,12 |
11,12 |
|
Penta D*
|
11,13 |
9,9 |
|
Penta E |
15,16 |
12,18 |

BMC was also analyzed with NGS by using the HID-Ion AmliSeq Identity Panel (Life Technologies, Milan, Italy). Bar-coded libraries were generated by using the Ion Xpress Plus Fragment Library Kit (Life Technologies) and Ion Xpress DNA Bar Coding Kit (Life Technologies) with adaptors. The panel selectively analyzed gene content by discriminating 120 SNPs, including 90 autosomal SNPs and 30 upper Y-Clade SNPs. Since both the donor and recipient were women, 30 upper Y-Clade SNPs were not used. Out of 90 SNPs, 32 sequence variations were observed and read number of variant sequence per read number of expected sequence was calculated to determine percent chimerism (
Fig. 1).
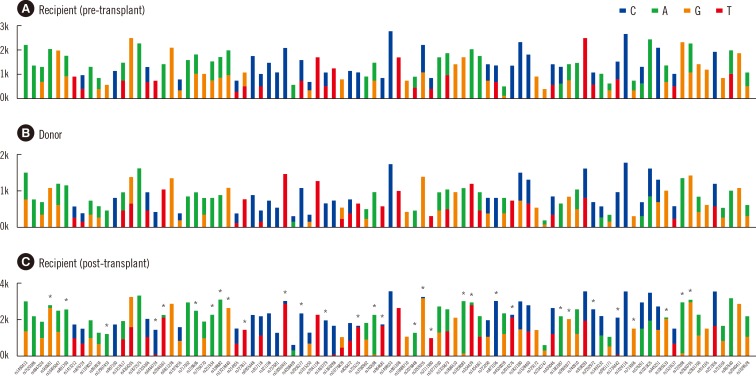 | Fig. 1
NGS chimerism analysis 41 days after liver transplant. Thirty-two of ninety SNPs discriminated between the donor and recipient. The average percent of donor DNA was 95.1%. Ninety SNPs are shown for (A) the recipient (peripheral blood, pre-transplant), (B) donor (peripheral blood), and (C) recipient (bone marrow, post-transplant) with mean coverage depth for each SNPs, 674, 1,609 and 1,080, respectively. The following markers were used for the chimerism calculation: rs560681, rs891700, rs12997453, rs6444724, rs2046361, rs159606, rs251934, rs338882, rs13218440, rs727811, rs10092491, rs2056277, rs1463729, rs735155, rs740598, rs964681, rs2076848, rs2269355, rs2111980, rs1058083, rs354439, rs873196, rs2016276, rs1382387, rs9905977, rs2292972, rs1736442, rs719366, rs1005533, rs722098, rs2830795, and rs987640.
*discriminating SNPs used in chimerism calculation.
Abbreviations: NGS, next generation sequencing; SNPs, single nucleotide polymorphisms.

|
The percentage of recipient DNA determined in mixed chimerism analyses were 5.6% (range 4.1-7.7%) by STR-PCR and 4.9% (range 2.5-6.7%) by NGS. The percent recipient DNA determined by NGS was equivalent to that determined by STR-PCR, indicating its applicability in chimerism analysis.
Pancytopenia after SOT is the presenting symptom of life-threatening pathologies, such as SOT-GVHD, post-transplant lymphoproliferative disorder, or unsuspected opportunistic infection [
6]. Although lymphocyte macrochimerism can occur transiently and decrease rapidly over time, usually within the third postoperative week [
7], this study illustrates an occurrence of BMC six weeks after liver transplantation, with 95.1% of the DNA originating from the donor. This high donor chimerism has been associated with liver transplants from deceased donors and recipients with hepatitis C virus [
3]. The relative amount of DNA from the recipient was 4.9%, which was too low to be reliably distinguished from background noise and could cause misinterpretation in cases of mixed chimerism [
8]. Here, we used NGS to quantify chimerism and confirmed the applicability of this method by comparing with conventional STR-PCR. NGS is an emerging, promising diagnostic tool for a number of clinical applications and forensic analyses [
9]. The present observations may have implications in the field of transplantation research and exhibit the diagnostic utility of this new technology.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download