Dear Editor,
Interferon (IFN)-γ release assay (IGRA) is a functional assay used to measure T cell responses to Mycobacterium tuberculosis-specific antigens in whole blood or blood-derived mononuclear cells [12]. The QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis, Carnegie, Australia) is a commercially available IGRA. Significant within-subject variability in QFT-GIT results has been reported in tuberculosis (TB) screening [34]. Variability in QFT-GIT results for the retesting of the same patient sample has been observed in a low TB-incidence setting. Twenty-eight (8%) of 366 patients showed results different from those of the initial QFT-GIT, and 24 (85.7%) of these 28 patients had an initial TB response between 0.25 and 0.80 IU/mL [5].
To explore the clinical impact of QFT-GIT results with borderline TB response in an intermediate TB-incidence setting, we specifically assessed the repeatability of QFT-GIT near cut-off points in routine laboratory practice. We prospectively included 748 samples referred for TB screening between March, 2013 and February 2014 in the Dong-A University Hospital, Busan, Korea. This study was approved by the Institutional Review Board of Dong-A University Hospital. The QFT-GIT was performed according to the manufacturer's instructions. A TB response between 0.25 and 0.80 IU/mL was adopted as a borderline range [5]. After the initial test, the residual samples with borderline range of TB response, were stored and retested. According to the manufacturer's interpretation criteria, we compared and analyzed the first and second results. Bland-Altman plots were used for quantitative results. Data analysis was performed by using MedCalc Software (ver. 12.6.1, MedCalc Software, Mariakerke, Belgium).
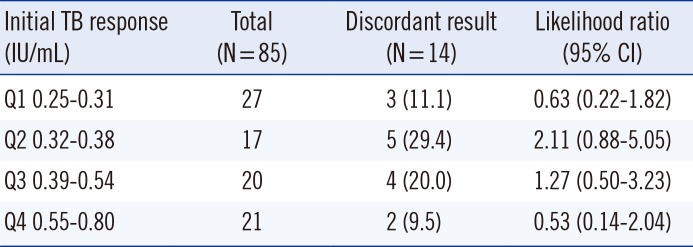
Eighty-five of the 748 patients showed a TB response in the range of 0.25-0.80 IU/mL. Among these 85 patients, 25 (29.4%) were diagnosed as having TB, 28 (33%) had suspected symptoms of TB, and 32 (37.6%) were examined by QFT-GIT to screen for latent TB infection. The median age was 56 yr, and 47 (55.3%) were males. The first QFT-GIT result was positive in 49 patients (57.6%), negative in 35 (41.2%), and indeterminate in 1 (1.2%). Seventy-one patients (83.5%) showed concordance between the results of the first and second QFT-GIT. Of the 14 patients with discordant results (14/85, 16.4%), three showed positive conversion, eight showed negative reversion, one with an indeterminate result in the first QFT-GIT showed a negative result in the second QFT-GIT, and two with positive results in the first QFT-GIT showed indeterminate results in the second QFT-GIT. The discordant results were most frequent (5/17, 29.5%) in the group with second-quartile TB response where the values included the cut-off value of 0.35 IU/mL. However, the likelihood ratio for subsequent discordant results was not statistically significant (P=0.48; Table 1). Bland-Altman plots of the differences in the first and second responses for nil, mitogen, and TB responses are shown in Fig. 1; the mean difference in the mitogen response was the highest.
The repeatability and reproducibility of QFT-GIT have important clinical considerations because the test uses a single cutoff point to distinguish between positive and negative results with one-time testing. The low positive result for TB response (0.35-0.59 IU/mL) could be supposed to revert to negative in the retest on the basis of inherent variability of the QFT-GIT using a linear mixed effects model [5]. The conversion and reversion may have occurred in patients undergoing serial testing, and this could result in unnecessary preventive therapy while screening for latent TB infection [67]. In this study, we observed that a considerable portion of the QFT-GIT results near the cut-off points showed discordance on retesting of the same sample. Thus, it might be worth introducing a borderline decision range for quantitative TB antigen response in QFT-GIT. In addition, we found that the mitogen response would be more dynamic than nil and TB responses, consistent with those of a previous study involving serial QFT-GIT assays in 299 patients [8].
Although we could not examine subjects with a TB response <0.25 IU/mL and >0.8 IU/mL, nearly two-thirds of the discordant results were observed between the cut-off points minus 0.03 IU/mL and plus 0.19 IU/mL for the TB response (0.32-0.54 IU/mL). This range considerably overlaps a low positive zone (0.35-0.59 IU/mL) defined by a previous study on QFT-GIT variability [5].
In conclusion, we suggest that to improve the clinical interpretation of QFT-GIT results, the results near the cut-off points in particular should be interpreted cautiously, and the establishment of a borderline decision range for quantitative TB antigen response in QFT-GIT should also be considered.
References
1. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. IGRA Expert Committee. Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection-United States, 2010. MMWR Recomm Rep. 2010; 59:1–25. PMID: 20577159.
2. Whitworth HS, Scott M, Connell DW, Dongés B, Lalvani A. IGRAs--the gateway to T cell based TB diagnosis. Methods. 2013; 61:52–62. PMID: 23296020.
3. Park JS, Lee JS, Kim MY, Lee CH, Yoon HI, Lee SM, et al. Monthly follow-ups of interferon-γ release assays among health-care workers in contact with patients with TB. Chest. 2012; 142:1461–1468. PMID: 22556318.
4. Ringshausen FC, Nienhaus A, Torres Costa J, Knoop H, Schlösser S, Schultze-Werninghaus G, et al. Within-subject variability of Mycobacterium tuberculosis-specific gamma interferon responses in German health care workers. Clin Vaccine Immunol. 2011; 18:1176–1182. PMID: 21593237.
5. Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med. 2013; 187:206–211. PMID: 23103734.
6. Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014; 27:3–20. PMID: 24396134.
7. Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, Cattamanchi A, et al. Reproducibility of interferon gamma (IFN-γ) release assays. A systematic review. Ann Am Thorac Soc. 2014; 11:1267–1276. PMID: 25188809.
8. Woo KS, Choi JL, Kim BR, Kim JE, Kim BG, Lee H, et al. Significance of interferon-gamma response to mitogen in serial QuantiFERON-TB Gold In-Tube assay of routine laboratory practice. Clin Chim Acta. 2014; 430:79–83. PMID: 24389051.
Fig. 1
Bland-Altman plots for the first-second response differences for nil (A), mitogen (B), and TB responses (C); the analysis showed mean differences of -0.02 IU/mL, -0.2 IU/mL, and 0.03 IU/mL, respectively. The lower and upper limits of agreement (mean difference±1.96 SD) were -0.5 IU/mL and 0.5 IU/mL for the nil response, -2.6 IU/mL and 2.2 IU/mL for the mitogen response, and -0.5 IU/mL and 0.6 IU/mL for the TB response, respectively. Mitogen1 indicated initial result for initial mitogen stimulation and mitogen2 indicated second result for second mitogen stimulation.
Abbreviation: TB, tuberculosis.
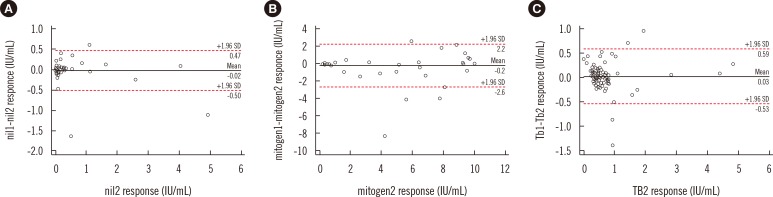
Table 1
Distribution of discordant results based on the initial TB response




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download