Dear Editor,
T lymphoblastic leukemia (T-ALL) comprises 10-15% of pediatric ALL cases and is known to be a clinically and genetically heterogeneous disease [1]. Recent studies have presented various cytogenetic abnormalities in T-ALL. The alterations often present as reciprocal translocations implicated in several transcriptional factor genes and lead to arrest during T-cell differentiation [12]. Although cytogenetic aberrations are known to be critical in leukemogenesis of many hematologic malignancies, the relationships between these aberrations and the prognosis are not well understood in T-ALL except for t(11;19), a mixed-lineage leukemia gene (MLL)-ENL fusion related to favorable prognosis [3]. Here, we report a very rare case of pediatric T-ALL with t(11;17)(q23;q21) involving MLL rearrangement, which has not been previously reported in Korea.
A 16-month-old boy was admitted to our hospital because of fever, cough, rhinorrhea, and poor oral intake. Physical examination revealed anemic conjunctiva, cervical lymph node enlargement, and splenomegaly. Chest radiography showed no abnormal findings. The complete blood count (CBC) results were as follows: hemoglobin, 8.2 g/dL; leukocytes, 41.3×109/L; platelets, 177×109/L; and reticulocyte count, 3.4%. The blood smear showed increased blasts (66%). The bone marrow (BM) specimen contained 65% blasts and showed a hypercellular pattern. Small-to-medium sized blasts were observed with condensed nuclear chromatin and an irregular nuclei margin. Immunophenotyping using the BM aspirate was brightly positive for CD2 (86%), cCD3 (91%), CD5 (91%), CD7 (92%), and CD34 (76%). Serum lactate dehydrogenase was increased to 638 IU/L. Cytogenetic studies on the BM revealed the karyotype of 46,XY,t(11;17)(q23;q21)[17]/46,XY[3] (Fig. 1A). The patient was diagnosed with T-ALL according to the 2008 WHO classification [4].
The patient began receiving induction chemotherapy comprised of prednisolone, vincristine, L-asparaginase, intrathecal cytosine arabinoside, and methotrexate. He seemed to respond to chemotherapy, with decreasing leukocyte counts and spleen size. However, the BM aspirate performed on the 7th day showed increased blasts to 69%. Moreover, interphase FISH analysis using an 11q23 break-apart probe revealed MLL rearrangement (158 of 200, 79%) (Fig. 1B). He was switched to more intensive induction chemotherapy including doxorubicin and cyclophosphamide. The patient achieved complete remission after 28 days of re-induction chemotherapy. Because of his high-risk features, the patient received cord blood stem cell transplantation. At the time of writing, he is alive and has been in a continuous complete remission state for 26 months.
MLL/11q23 rearrangement is intricate in many hematologic malignancies such as ALL, AML, and MDS. The 11q23 translocation, which is rarely identified in T-ALL, is a distinct subtype characterized by differentiation arrest at an early stage of thymocyte differentiation [5]. Since MLL fusion partners are also known to have an important role in leukemogenesis, recent efforts to unravel MLL fusion partners using long-distance inverse-PCR (LDI-PCR) as compensatory diagnostic method have been implemented by using current molecular diagnostic tools [67]. Not only scarce MLL rearrangement but also few fusion genes are involved in T-ALL, despite that 79 different MLL fusion partner genes have been identified in other hematologic malignancies [68].
When balanced t(11;17)(q23;q21) involving MLL rearrangement was searched on the molecular level, three different fusions with MLL at 11q23 were generated by proximally clustered genes at the same chromosomal loci: MLLT6/AF17, ACACA, and LASP1 at 17q21 [8]. Thus far, MLL with each of the other three fusion partner genes at 17q21 has been identified mainly in AML and rarely in ALL [8]. Unfortunately, the fusion partner gene in this case could not be confirmed by using LDI-PCR because of specimen problems. A literature search revealed only two cases regarding t(11;17)(q23;q21) in adults with T-ALL [910] (Table 1).
Prior studies have suggested that immunophenotypic markers are correlated with genetic alterations in pediatric acute leukemia [2]. The immunophenotype of leukemic cells in our patient could be classified into the pre-T stage and immature stage according to the European Group for the Immunological Characterization of Leukaemias and T-cell receptor classification system [2]. The immature stage of T-ALL was reported with respect to poor outcome and the lack of a common genetic lesion [12]. In several studies, MLL-rearranged T-ALL overexpressed multiple HOX, which are transcription factors that initiate T-cell differentiation [5].
Additional cases involving genetic profiling of t(11;17)(q23; q21) involving MLL rearrangement will help assess whether this rare fusion impacts the disease progression and reveals prognostic information.
References
1. Patrick K, Vora A. Update on biology and treatment of T-cell acute lymphoblastic leukaemia. Curr Opin Pediatr. 2015; 27:44–49. PMID: 25502893.
2. Meijerink JP. Genetic rearrangements in relation to immunophenotype and outcome in T-cell acute lymphoblastic leukaemia. Best Pract Res Clin Haematol. 2010; 23:307–318. PMID: 21112032.
3. Pui CH, Schrappe M, Ribeiro RC, Niemeyer CM. Childhood and adolescent lymphoid and myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2004; 118–145. PMID: 15561680.
4. Borowitz MJ, Chan JKC. T lymphoblastic leukaemia/lymphoma. In : Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008. p. 176–178.
5. Ferrando AA, Armstrong SA, Neuberg DS, Sallan SE, Silverman LB, Korsmeyer SJ, et al. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003; 102:262–268. PMID: 12637319.
6. Meyer C, Hofmann J, Burmeister T, Gröger D, Park TS, Emerenciano M, et al. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013; 27:2165–2176. PMID: 23628958.
7. Yang JJ, Marschalek R, Meyer C, Park TS. Diagnostic usefulness of genomic breakpoint analysis of various gene rearrangements in acute leukemias: a perspective of long distance- or long distance inverse-PCR-based approaches. Ann Lab Med. 2012; 32:316–318. PMID: 22779077.
8. Strehl S, König M, Meyer C, Schneider B, Harbott J, Jäger U, et al. Molecular dissection of t(11;17) in acute myeloid leukemia reveals a variety of gene fusions with heterogeneous fusion transcripts and multiple splice variants. Genes Chromosomes Cancer. 2006; 45:1041–1049. PMID: 16897742.
9. Harrison CJ, Cuneo A, Clark R, Johansson B, Lafage-Pochitaloff M, Mugneret F, et al. Ten novel 11q23 chromosomal partner sites. European 11q23 Workshop participants. Leukemia. 1998; 12:811–822. PMID: 9593286.
10. Beverstock GC, Hoekman K, Kluin-Nelemans JC, Wienhofer E. Reciprocal translocation, t(11;17)(q23-25;q21), in a patient with an immature ALL. Cancer Genet Cytogenet. 1986; 22:83–87. PMID: 3456831.
Fig. 1
Bone marrow karyogram at diagnosis and interphase FISH analysis after induction chemotherapy. (A) G-banded karyogram showing t(11;17)(q23;q21). The arrows denote the rearranged chromosomes. (B) FISH analysis using 11q23 break-apart probe revealed MLL rearrangement (158/200, 79%). Separation of a green and a red signal was observed.
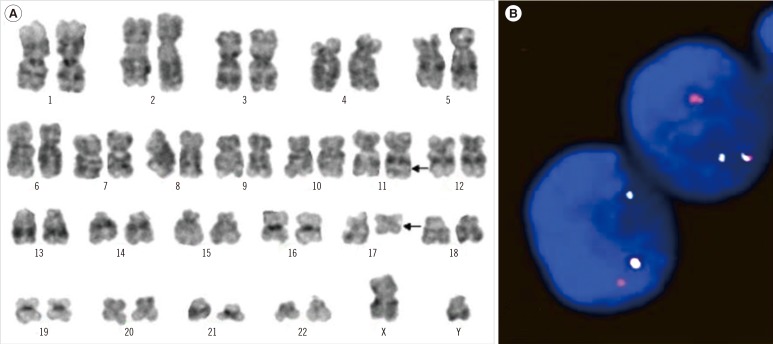
Table 1
Reported cases of T-ALL with t(11;17)(q23;q21)
| Case No. | Age/sex | Leukocytes (×109/L) | Hb (g/dL) | Platelets (× 109/L) | Blasts in PB (%) | Karyotypes | Immunophenotypes | OS (mo) | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 yr/F | 0.05 | 8.4 | 0.08 | 68 | 46,XX,t(11;17) (q23-25;q21) | CD7(+), OKT10(+), CD1(-), CD3(-), CD4(-), CD8(-), CD10(-), CD19(-), anti-HLA-DR(-), 63D3(-), and TdT(-)* | > 14.5 | the Netherlands | 9 |
| 2 | 49 yr/M | ND | ND | ND | ND | 46,XY,t(11;17) (q23;q21) | ND | 11.6† | EU‡ | 10 |
| 3 | 16 mo/M | 41.3 | 8.2 | 177 | 66 | 46,XY,t(11;17)(q23;q21)[17]/46,XY[3] | CD2 (86%), cCD3 (91%), CD5 (91%), CD7 (92%), and CD34 (76%) | >26.4† | Korea | Present case |
*The results were performed with immunohistochemical stain; †The patients received bone marrow transplantation; ‡The European 11q23 Workshop participants.
Abbreviations: T-ALL, T lymphoblastic leukemia; PB, peripheral blood; OS, overall survival; F, female; CD, cluster of differentiation; TdT, terminal deoxynucleotidyl transferase; M, male; ND, no data; EU, European Union; mo, months.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download