Sepsis is life-threatening organ dysfunction from a dysregulated host response to infection [
1]. Among sepsis-induced complications, the development of hemodynamic instability or cardiac dysfunction is a major risk factor of in-hospital mortality. For early detection of sepsis-induced cardiac dysfunction (SICD), immediate echocardiography is crucial but may be delayed owing to inability of bedside skilled staff or high cost. For early diagnosis and decision-making in this critical setting, biomarkers like high-sensitivity C-reactive protein (hs-CRP) and procalcitonin (PCT) have been suggested to indicate septic processes [
2]. A multi-marker or complementary approach with other biomarkers related to SICD might be helpful to determine the appropriate therapy for cardiovascular support.
Among the emerging cardiac inflammatory biomarkers, soluble suppression of tumorigenicity 2 (sST2), a member of the Toll-like/interleukin (IL)-1 receptor superfamily, seems promising. Clinical studies revealed the diagnostic value of sST2 for acute myocardial infarction, acute heart failure, sepsis, and trauma [
345]. Recently, we demonstrated the prognostic role of sST2 in sepsis and its association with the cardiovascular subscore of sepsis-related organ failure assessment (SOFA) scores [
6]. However, the cardiac subset of SOFA elements consist of only mean arterial pressure and use of a vasopressor, which are too limited to reflect SICD alone [
7]. In this study, we hypothesized that SICD would result in enough mechanical stress to release sST2, and sST2 may reflect systolic or diastolic dysfunction as measured by Doppler echocardiography in sepsis patients. We explored whether sST2 correlates with echocardiographic findings and other sepsis biomarkers or not.
A retrospective study was conducted in two tertiary referral hospitals: Sant'Andrea Hospital (SAH), Rome, Italy and Konkuk University Medical Center (KUMC), Seoul, Korea. The hospital echocardiographic database including medical/surgical intensive care units, general wards, and emergency department was searched for the period from November 1, 2013 to May 31, 2014 (SAH) and November 1, 2013 to August 31, 2014 (KUMC). This study was conducted separately from our previous study [
6] that has the overlapping study period, using a different study protocol. This study protocol involved reviewing medical records on clinical, laboratory, and echocardiographic data. The protocol met the criteria of the Declaration of Helsinki and was approved by the Institutional Review Board of each hospital. Informed consent was obtained from each patient in SAH for the biomarker measurement and review of medical records; in KUMC, it was exempted for this retrospective study.
Among the eligible adult patients (≥18 yr) who had available echocardiographic data, only the patients diagnosed as having sepsis and with relevant laboratory data were included. Although echocardiography is not a routine practice for every septic patient, the decision to perform echocardiography was made by the attending clinicians when the patient had hemodynamic instability, dyspnea, and/or abnormal radiographic findings on a chest X-ray. Each patient was considered eligible only when it was his or her first time in the hospital due to sepsis. Because the patients were searched not by the whole hospital database but by the echocardiographic database, information on how many patients were diagnosed as having sepsis during the study period was not available for this study. The following exclusion criteria were applied in the following order: patients' age <18 yr; absence of routine laboratory data for PCT and/or hs-CRP that were measured concomitantly with echocardiography (on the same day or within 24 hr); and absence of concomitantly obtained frozen samples, acute coronary syndrome, and documented history of symptomatic heart failure.
Recently, the diagnosis of sepsis was redefined according to the most up-to-date consensus definition [
1]. Among the eligible 136 patients, nine patients were excluded owing to acute coronary syndrome (N=2), documented history of symptomatic heart failure (N=4), and the new sepsis definition (N=3) (
Supplemental Data Fig. S1). We enrolled 127 patients (110 patients from KUMC and 17 patients from SAH), consisting of 95 patients with sepsis (74.8%) and 32 patients with septic shock (25.2%) (
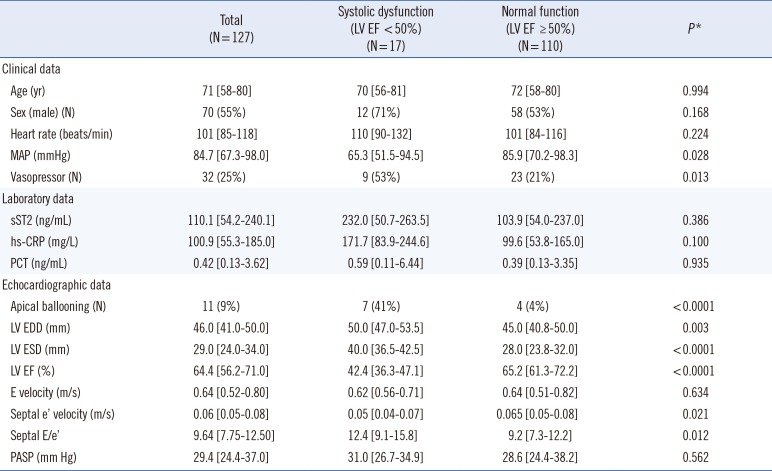
Table 1). The median age was 71 yr (range, 58-80 yr), and 55% of patients were male. No statistical difference was observed between the patients from KUMC and SAH, in terms of age, sex, use of vasopressor, mean arterial pressure, left ventricular (LV) ejection fraction (EF), or sST2 concentrations. Their underlying medical conditions were: pneumonia (N=47), hematologic immunocompromised state (N=19), urinary tract infection (N=15), hepatobiliary (N=14), cerebral (N=12), solid organ malignancy (N=8), bone or spine (N=6), gastrointestinal (N=5), and others (N=1, mucormycosis).
Serum PCT and hs-CRP concentrations were measured according to the manufacturers' instructions [
1]. In KUH, PCT was measured by an Elecsys BRAHMS PCT electrochemiluminescence assay (BRAHMS, Hennigsdorf, Germany) in a Roche Cobas system (Roche Diagnostics, Basel, Switzerland), and in SAH PCT was measured by using a BRAHMS PCT sensitive Kryptor immunofluorescent assay system (BRAHMS). hs-CRP was measured by using the BN II Nephelometer (Dade Behring Ins, Newark, DE, USA). Frozen sera stored at -70℃ were thawed once for sST2 measurement. Briefly, sST2 concentrations were measured in duplicate by using an enzyme-linked immunosorbent assay, the Presage ST2 assay (Critical Diagnostics, San Diego, CA, USA) [
8]. The maximum total CV (cumulative calculation) of sST2, PCT, and hs-CRP assays in both laboratories were 3%, 3.2%, and 3.3%, respectively.
The transthoracic Doppler echocardiogram records on the Cardiology PACS system (INFINITT healthcare, Seoul, Korea) were reviewed. LV dimensions were measured by M-mode, tracing both end-diastolic and end-systolic period. LV systolic function was evaluated with EF by two-dimensional images using the modified Simpson's method [
9]. LV diastolic function was assessed with the ratio of pulsed-wave Doppler-derived early transmitral inflow velocity (E velocity) and mitral septal annular tissue Doppler-derived early diastolic velocity (e' velocity) (E/e' ratio) [
10]. Resting pulmonary arterial systolic pressure (PASP) was assessed via the tricuspid regurgitation jet peak velocity using the simplified Bernoulli equation with an assumption of right atrial pressure [
11]. Two experienced cardiologists reviewed the images for apical ballooning, LV outflow dynamic obstruction, or right ventricular dysfunction [
12]. For discrepant opinions between them, a consensus was drawn throughout their discussion.
We performed statistical analysis using dBSTAT (DBSTAT Version 5. Chuncheon, Korea). Data were expressed as a median (interquartile range) for continuous variables and as a number (percentage) for categorical variables. We compared clinical, laboratory, and echocardiographic data in patients with or without sepsis-induced LV systolic dysfunction (EF <50%). Correlation between sST2 and cardiovascular or inflammatory markers was assessed by using Pearson's correlation coefficient (95% confidence interval). Mann-Whitney U test was used for continuous variables, and Fisher's exact test for categorical variables. Obtained P values were not adjusted for multiple comparisons and are therefore descriptive only.
Sepsis-induced LV systolic dysfunction was observed in 17 patients (13.4%) (
Table 1). Except for E velocity and PASP, the other echocardiographic data showed significant differences between the patients with and without LV systolic dysfunction. Although median sST2, hs-CRP, and PCT concentrations tended to be higher in the systolic dysfunction group, unadjusted
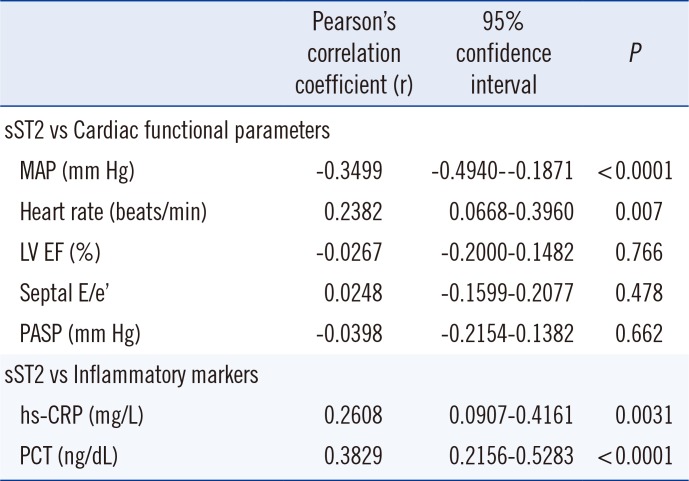
P values did not indicate a meaningful difference. sST2 concentration correlated moderately with hs-CRP and PCT concentrations; it also correlated moderately with the hemodynamic parameters of blood pressure and heart rate. No correlation was observed between sST2 concentration and LV EF, septal E/e', or PASP (
Table 2).
The heart is one of the most frequently affected organs in sepsis; up to 60% of patients with septic shock develop LV EF <45% during the first three days [
13]. The prevalence or degree of LV dysfunction depends on the timing and severity of sepsis. In this study, depressed LV systolic function was observed only in 13.4% of patients, possibly owing to the relatively small portion of patients with septic shock (25.2%). In addition, the examination timing within 24 hr after blood collection for PCT and hs-CRP measurement (approximately the time point when the diagnosis of sepsis was established) may include the compensatory hyperdynamic contractile period. During early phases of sepsis, the LV EF is high (>55%) with insufficient cardiac preload due to high vascular permeability and low vascular tone; however, during the later phase with fluid loading, EF decreases [
14]. Therefore, serial echocardiography Doppler monitoring is warranted.
To the best of our knowledge, this is the first study to compare sST2 concentration with concomitant Doppler echocardiographic findings in septic patients. We speculated that sepsis-induced acute LV systolic or diastolic dysfunction, with decreased LV EF or elevated LV filling pressure, would be enough mechanical stress to release sST2. However, our findings did not support this hypothesis, similarly to the recent report that revealed no correlation between inflammatory cytokines and myocardial dysfunction in septic shock [
15]. Sepsis itself may trigger sST2 release as an inflammatory mediator, covering up any sST2 upregulation from myocardial mechanical strain. The median sST2 concentration was 110 [54.2-240.1] ng/mL, and most patients (118/127 patients, 92.9%) exhibited higher sST2 concentrations than the reference value (≤35 ng/mL) [
8]. These results are consistent with our previous study on patients with stress cardiomyopathy in a non-cardiac medical intensive care unit; initial sST2 concentration did not predict stress cardiomyopathy, but the 48-hr follow-up sST2 concentration (persistently or newly increased sST2) did [
16]. A limitation of our study is that we only performed a single sST2 measurement. A short-term follow-up of sST2 concentration is necessary to delineate its cardiac-specific role. More specifically, an algorithm combining sST2 and echocardiography may be proposed: sST2 and echocardiography can be evaluated in the early period of sepsis (within 24 hr of diagnosis) and followed up after 48 hr of the initial evaluation.
Although we excluded the patients with documented history of symptomatic heart failure, it is possible that some patients had undiagnosed/asymptomatic heart failure or new onset of symptomatic failure. This is another limitation of our study. Moreover, we could not include other cardiac biomarkers in our retrospective analysis. Although cardiac troponins and natriuretic peptides have been studied in relation to echocardiographic findings, they did not show satisfactory results [
1718]. Absence of comparison data with the other cardiac biomarkers may be another limitation of this study.
It is unclear whether elevated sST2 in acute heart failure is produced in cardiac myocytes or elsewhere. Intuitively, sST2 protein is upregulated by myocardial stress in cultured myocytes [
19], but other studies did not show elevated myocardial sST2 expression during heart failure [
20]. In our sepsis population, sST2 is mainly a marker of inflammation, and even in heart failure, the major source of circulating sST2 is likely not the heart. Therefore, we speculate the possibility of non-myocardial or systemic vascular production of sST2 is triggered by altered myocardial load or low blood pressure in sepsis (
Table 2).
In conclusion, sST2 might have a role as a biomarker of shock or inflammation in sepsis. A single measurement of sST2, however, cannot reflect sepsis-induced LV systolic or diastolic function evaluated by Doppler echocardiography. Serial measurement of sST2 along with echocardiography Doppler monitoring would be necessary to better delineate the role of sST2 as a marker of SICD.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download