See the reply "Reply to the Letter to the Editor by Dr. Chirumbolo" in Volume 36 on page 498.
Dear Editor,
Zehwan Kim et al, [1] presented a protocol to assay basophil activation by flow cytometry (FC) that included a phenotyping panel with CD123 and CD193 (CC chemokine receptor-3 [CCR3]). This method for the electronic capture of basophils in whole blood, never published before,raises some interesting questions.
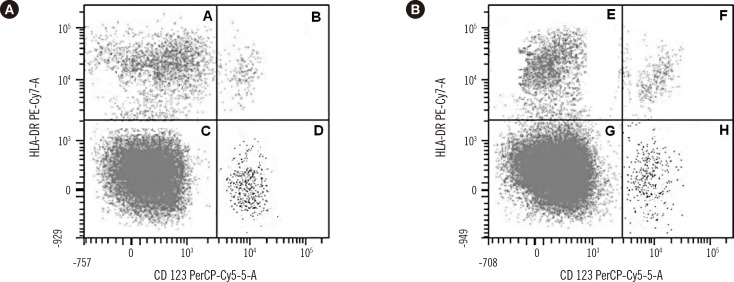
CCR3 (CD193), the eotaxin receptor, is commonly present in CD3-expressing T-cells [23] and it would be interesting to assess whetherthe basophil gating also included CD3pos cells as contaminants. Furthermore, dendritic cells express CD123, the α-chain of the interleukin (IL)-3 receptor [4] (see also Fig.1, panels B and F). Therefore, without additional markers to exclude other cells, the CCR3pos/CD123pos gate may not include only purified basophil cells [5]. Introducing additional markers is therefore recommended. For example, searching for cells that do not express the HLA-DR marker in gate 3 of reference [1] could be useful in capturing basophils (see also Fig. 1). The use of markers expressed by immune cells other than basophils may fundamentally distort the total basophil count calculated as dot events within the reported FC gate and, consequently, the percentage of cells expressing CD63 upon cell activation [6]. Actually, the authors evaluated about 2,000 basophils/100 µL [1], and, with a white blood cells (WBC) normal count of 45,000–50,000 in a 100-µL volume, basophils (1%) should be as high as 450–500 within the gate, while a value of 2,000 basophils might be retrieved with WBC total counts of 2,000,000/100 µL, as it yet occurs in newborns. Fig. 1 reports cells captured in the whole blood of healthy donors and amounting to ≤500 basophils (Fig. 1). In one of our previousstudies, we captured ≥1,500 basophils/gate with a CD123/HLA-DR protocol, but only if we used a buffy coat pooled sample [7]. Bias may be significant only for cells expressing CCR3, because basophils express CD123 in a bright manner. Therefore, no cell with the CD123neg/CCR3pos phenotype would be detected in the basophil gate. Moreover, CD123bright/CCR3dim cells might be confused with activated basophils, and additional markers (CD14/CD11c) might be needed to assess whether monocytes were included in gate 2 [1].
Aside from the possibility of contaminating non-basophilic cells present in gate 3 in the paper [1], the authors identified the need to add the CD63 marker to the basophil activation test (BAT), as CD203c expression is dependent on primed basophils [18]. Interestingly, cut-off values higher than 100% have been described for CD203c in primed basophils. This corresponds to the lowest sensitivity value for this marker in gate 3, indicating a possible bias in the cut-off thresholds [18]. This should lead the authors to conclude that CD203c has to be evaluated in non-primed basophils and that CD63 should be reported as a fundamental marker to assess basophil activation [1].
The suggestion by the authors that CD123 may be sufficient to capture basophils as CD123pos or CD123bright cells may not be correct because plasmacytoid dendritic cells (DCs) are notoriously CD123bright (Fig. 1, panels B and F), even though they do not express CD203c. Although the amount of circulating DCs may be lower than that of basophils, the calculated fluorescence means and activation percentages, particularly for CD63, may be biased because of an incorrect evaluation of dots within the gate. Different approaches might be used to address this technical issue (see Fig.1). It might be argued that CD63 and CD203c should be expressed together and fully agree for the best BAT performance; however, in order to prevent priming, cells need to be handled with special care. For example,cells must be processed within the first 4 hr following peripheral blood withdrawal and in an ice bath (resting cells) [789]. The authors raised the concern that in chronic urticaria (CU), circulating IL-3 may create false positives in CD203c expression; however, they did not address this issue in resting basophils from CU subjects with an atopic response, as the authors suggest using CD203c alone to assay basophil activation [1].
In a more general way, the suggestion addressed with this manuscript, asks the authors for further insights. Basophils are known to express CD123 highly and express CD203c exclusively; therefore, these two membrane molecules might be the best markers to capture and purify basophils in the FC channels. However, the main concern in this protocol is the use of markers that show altered patterns of expression upon activation. Although CD123 is expressed at a "maximal" saturation level and activation does not upregulate CD123 following a stimulus, CCR3, CD203c, and CD63 membrane expression depends on IgE-mediated or non-mediated stimulation, and therefore may alter the gate if used in a phenotyping protocol [2].
References
1. Kim Z, Choi BS, Kim JK, Won DI. Basophil markers for identification and activation in the indirect basophil activation test by flow cytometry for diagnosis of autoimmune urticaria. Ann Lab Med. 2016; 36:28–35. PMID: 26522756.
2. Chirumbolo S, Ortolani R, Vella A. CCR3 as a single selection marker compared to CD123/HLADR to isolate basophils in flow cytometry: some comments. Cytometry A. 2011; 79:102–106. PMID: 21265004.
3. Monneret G. CCR3 for basophil activation test: a necessary but insufficient step. Clin Exp Allergy. 2010; 40:953. author reply 954. PMID: 20557552.
4. Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004; 5:1219–1226. PMID: 15549123.
5. Beaulieu S, Robbiani DF, Du X, Rodrigues E, Ignatius R, Wei Y, et al. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J Immunol. 2002; 169:2925–2936. PMID: 12218106.
6. Chirumbolo S. Basophil activation test in allergy: time for an update? Int Arch Allergy Immunol. 2012; 158:99–114. PMID: 22269476.
7. Chirumbolo S, Vella A, Ortolani R, De Gironcoli M, Solero P, Tridente G, et al. Differential response of human basophil activation markers: a multi-parameter flow cytometry approach. Clin Mol Allergy. 2008; 6:12. PMID: 18925959.
8. Chirumbolo S. The use of IL-3 in basophil activation tests is the real pitfall. Cytometry B ClinCytom. 2011; 80:137–138. author reply 139.
9. Chirumbolo S. CD203c in patients with cystic fibrosis and allergy to Aspergillus fumigatus. J Allergy Clin Immunol. 2016; 137:969. PMID: 26782973.
Fig. 1
Flow cytometry showing dot plots of basophils collected from two representative healthy blood donors. Basophils were gated according to reference 7. Panels A, E: T and B lymphocytes; B, F: plasmacytoid dendritic cells; C, G: monocytes; D, H: basophils. Number of gated CD123bright/HLADRneg basophils: 504 and 523, respectively. For further information, see references 2 and 7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download