Abstract
Background
Rapid detection of carbapenemase-producing gram-negative bacilli (GNB) is required for optimal treatment of infected patients. We developed and assessed a new disk carbapenemase test (DCT).
Methods
Paper disks containing 0.3 mg of imipenem and bromothymol blue indicator were developed, and the performance of the DCT were evaluated by using 742 strains of GNB with or without carbapenemases.
Results
The paper disks were simple to prepare, and the dried disks were stable at -20℃ and at 4℃. The DCT detected 212 of 215 strains (98.6% sensitivity with 95% confidence interval [CI] 96.0-99.5%) of GNB with known class A (KPC and Sme) and class B (NDM, IMP, VIM, and SIM) carbapenemases within 60 min, but failed to detect GES-5 carbapenemase. The DCT also detected all two Escherichia coli isolates with OXA-48, but failed to detect GNB with OXA-232, and other OXA carbapenemases. The DCT showed 100% specificity (95% CI, 99.2-100%) in the test of 448 imipenem-nonsusceptible, but carbapenemase genes not tested, clinical isolates of GNB.
Carbapenem-resistant gram-negative bacilli (GNB) have emerged and spread worldwide. Although carbapenem resistance can be due to porin loss or enhanced drug efflux, acquired carbapenem resistance due to carbapenemase production is a more serious problem, because the resistance can be transferred horizontally, even to genetically unrelated species. The time-consuming modified Hodge test was the only test recommended by the CLSI for the phenotypic detection of KPC-type carbapenemases [1]. In 2015, the rapid Carba NP test was added to the CLSI recommendations [234]. The Blue Carba test is a modification of the Carba NP test, which uses the pH indicator bromothymol blue (BTB) and the less costly antibacterial Tienam 500 (Merck Sharp & Dohme, Kenilworth, NJ, USA) and eliminates the bacterial lysis procedure [5]. Both the Carba NP and Blue Carba tests require an adjustment of imipenem substrate pH, and both tests are performed in liquid phase in microtiter plates or microtubes. Besides the widely used nitrocefin disk method for detection of various β-lactamases, a disk method with benzylpenicillin and cephaloridine plus clavulanic acid substrates has been used briefly to detect extended-spectrum β-lactamases [6].
The aim of our present study was to develop a rapid and simple disk test, based on detection of the acid formed from hydrolysis of imipenem by the increasingly prevalent GNB that produce class A KPC-type and class B NDM-type carbapenemases as well as the already prevalent metallo-β-lactamases (MBLs).
To assess the performance of the Disk carbapenemase test (DCT), a total of 742 strains of GNB were used consisting of 287 reference strains and clinical isolates with known carbapenemases, and 448 imipenem-non-susceptible, but carbapenemase gene not tested isolates. PCR detection of carbapenemase genes was performed by using primers and test conditions as described previously: blaKPC-2-like and blaGES-5 [7], blaNDM-1-like [8], blaIMP-1-like, blaVIM-2-like, and blaSIM-1-like [9], blaOXA-23-like and blaOXA-51-like [10], and blaOXA-48-like [11].
Injectable imipenem (Prepenem; JW Pharmaceutical, Seoul, Korea), containing 530 mg of imipenem (0.462 of total weight), 532 mg of cilastatin sodium, and 20 mg of sodium bicarbonate, were used to prepare the imipenem-incorporated substrate disks. Based on preliminary tests, disks containing 0.3 mg of imipenem were prepared by using a 15 mg of imipenem (32.46 mg of Prepenem) per 1,000 µL solution. To the weighed amount of imipenem-cilastatin powder, a 12 mg/mL NaCl solution (80% of total solution volume) and a 2mM ZnSO4 (Sigma-Aldrich, St. Louis, MO, USA) solution (10% of total solution volume) were added, and the mixture was immersed in a 43℃ water bath for about 5 min to allow the powder to dissolve. A 0.8% BTB (Yakuri Pure Chemical, Osaka, Japan) solution was prepared by dissolving 0.8 g of the powder in 30 mL of ethanol and adding 70 mL of distilled water (DW). And then the solution (10% of total solution volume) was added to the imipenem and ZnSO4 solution. The resulting solution was at pH 7.0 without adjustment. While held at 43℃, 20 µL aliquots of the imipenem solution were dispensed to 6-mm-diameter and 0.6-mm-thick empty paper disks (Toyo Roshi Kaisha, Tokyo, Japan). The disks on the bottom of plates were dried by placing them in a 35℃ incubator with mechanical air circulation for 1 hr. The top side of the disks retained a green color after drying. The disks were stored in airtight microfuge tubes without desiccant at both 4℃ and -20℃ and were stable for at least 6 months. The mean inhibition zone diameter for Pseudomonas aeruginosa ATCC 27853 decreased only 2 mm from 35 mm, and the rapidity of the DCT for the four control strains with KPC-2, NDM-1, IMP-1, and VIM-2 remained similar (data not shown).
To determine carbapenemase production, the bacterial isolates were subcultured overnight on Mueller-Hinton agar (Asan Pharmaceutical, Seoul, Korea), which were prepared by using dry product from Remel (Thermo Fisher Scientific, Waltham, MA, USA). Increasing the amount of growth transfer by colony sweeping resulted in a faster positive DCT. Therefore, to compare the speed of positive reactions for different carbapenemases, a relatively constant amount of growth was transferred. The disk was placed green topside down on a confluent growth and pressed gently by using forceps. Then, the disk was immediately lifted and laid green side up by touching with 20 µL of DW, which was dropped on the bottom of a Petri dish, making it easier to release the clinging disk from the forceps. A false-positive reaction resulted without the addition of DW. The plate lid was replaced to prevent drying, and the color change of the disk was observed at room temperature. The carbapenemase-negative control strains, P. aeruginosa and Acinetobacter baumannii, were used to aid in the reading of the reaction.
Inhibition of KPC enzyme activity was tested by using a 1,000 µg/20 µL tazobactam (Sigma-Aldrich) solution in DW. As an inexpensive alternative, a 4.5g piperacillin-tazobactam injection (Jeil Pharmaceutical) was used, which contained 4g of piperacillin, 500 mg of tazobactam, 2g of dextrose, 200 mg of sodium citrate, and 1 mg of EDTA disodium. Inhibition of MBL was tested by using a 60 mM EDTA solution. Inhibitor solutions in a 20-µL volume were dispensed into the bottom of a Petri plate along with the same volume of DW. A heavy lawn of the test organism was transferred to three DCT disks that were then individually placed on a 20-µL aliquot of DW and the two different inhibitor solutions. The presence of significant inhibition of color development in a disk was considered positive.
Immediately after the transfer of colonies, the color of the DCT disks was typically green, but was a more alkaline greenish blue with the Acinetobacter spp. A carbapenemase-positive reaction started with a greenish yellow color that progressed to yellow, and even to orange yellow (Fig. 1).
The sensitivity of the DCT was determined by using reference strains and clinical isolates with known carbapenemases (Table 1). Typically, KPC-producing isolates started to show a positive reaction immediately after transfer of the organism to the disk. All 35 Enterobacteriaceae isolates with molecular class A carbapenemase (34 isolates with KPC and one with Sme-1) showed positive reactions within 10 min (Table 1), whereas five GES-5-producing isolates did not show a positive result until 90 min (data not shown).
All 21 NDM-producing Enterobacteriaceae and Acinetobacter spp. isolates showed positive results within 15 min. Seventy-three of 74 IMP-type MBL-producing Pseudomonas spp. and Acinetobacter spp. showed a positive reaction within 10 min. But one reference isolate with IMP-7 showed unreliable results: a positive reaction at 60 min or negative results in repeat tests (Table 1).
The positive reaction of VIM-2-like MBL-producing Pseudomonas spp., especially P. putida, was slower than that of the IMP-1-like MBL-producing isolates. Seventy-eight of 80 isolates showed a positive reaction in ≤50-60 min. The remaining two strains, the VIM-1- and VIM-3-producing reference P. aeruginosa, showed slow positive or negative reactions depending on repeat tests. Five SIM-1-like enzyme-producing isolates of Acinetobacter spp. showed positive reactions within 20 min. The DCT showed an overall sensitivity of 98.6% (212 of 215 isolates, 95% confidence interval [CI]: 96.0-99.5%) in the detection of acquired MBLs and class A carbapenemases (Table 1).
Only two Escherichia coli isolates with OXA-48 were DCT positive within 10 min, whereas the remaining 21 E. coli and Klebsiella pneumoniae isolates with OXA-232 were DCT negative. None of the 49 A. baumannii isolates, including the 44 with an OXA-23-like gene, were DCT positive (Table 1).
Repeat tests showed that inherent MBL producers, one isolate each of Stenotrophomonas maltophilia with L1 and Pseudomonas otitidis with POM-1, showed slow positive reactions within 80-90 min and 11-20 min, respectively (data not shown).
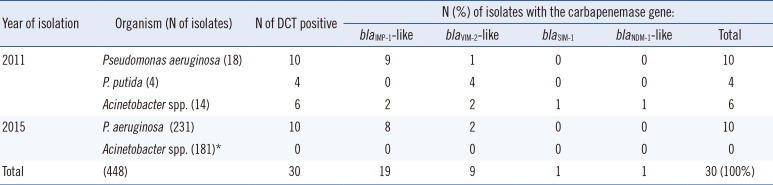
To determine the DCT specificity, imipenem-non-susceptible, but carbapenemase genes not tested isolates were used. Among the 36 isolates collected in 2011, 14 and six Pseudomonas spp. and Acinetobacter spp. isolates, respectively, were DCT positive. Subsequently, MBL genes were detected in all of them (Table 2). In routine testing in 2015, 10 of 231 consecutive P. aeruginosa isolates were DCT positive and blaIMP-1-like or blaVIM-2-like genes were detected in all of them, indicating 100% specificity (95% CI: 99.2-100.0%) for the DCT (Table 2). However, none of the 181 imipenem-non-susceptible Acinetobacter isolates in 2015 were DCT positive. Among these isolates, both blaOXA-51-like and blaOXA-23-like alleles were detected in all of the 51 randomly selected isolates, indicating that a majority of the current imipenem-non-susceptible Acinetobacter spp. isolates in our institute are OXA-23-like enzyme-producing A. baumannii (data not shown).
Inhibition tests with 1,000 µg of tazobactam (using piperacillin-tazobactam) and 20 µL of 60 mM EDTA per disk showed that for all 18 K. pneumoniae isolates with KPC-type enzymes and 11 Enterobacteriaceae and one Acinetobacter guillouiae isolates with NDM-type enzymes, the test interpretation was possible within 2-10 min depending on the isolate (data not shown). In a few isolates with the KPC enzyme, only partial inhibition by tazobactam was observed, necessitating a comparison of disk color with those without the inhibitor. A majority of the isolates with NDM enzymes showed complete EDTA inhibition (Fig. 1B). EDTA inhibition was detected within 5-20 min in one E. coli strain with a VIM-2-like enzyme, and one each of Acinetobacter junii and P. aeruginosa with an IMP-1-like enzyme, and one P. aeruginosa with a VIM-2-like enzyme; however, the test required 15-60 min of observation time for seven P. putida isolates with VIM-2-like enzymes.
In the preliminary test, the DCT-positive reaction was more distinct with BTB (transition range pH 6.0-7.6) than with bromocresol purple (transition range pH 5.2-6.8), and cell lysis using Bacterial Protein Extraction Reagent (B-PER; Thermo Scientific, Pierce Biotechnology, Rockford, IL, USA) did not enhance the reaction (data not shown).
Without adjustment, 1,000 µL of solutions with imipenem 10 mg (21.64 mg of Prepenem for 0.2 mg/disk), 15 mg (32.46 mg of Prepenem for 0.3 mg/disk), and 20 mg (43.29 mg of Prepenem for 0.4 mg/disk), showed pH values of 6.8, 7.0, and 7.1, respectively. We decided to use 0.3 mg imipenem disks for this study because the 0.2 mg disks showed a faster positive reaction but the colors faded more rapidly, whereas the 0.4 mg disks showed a slower positive reaction. In our study, all five GES-5-producing isolates did not show a positive result until 90 min (data not shown). It was reported that all four GES-5-positive isolates were Carba NP test negative [12].
Only one each of Pseudomonas strains with IMP-7, VIM-1, and VIM-3 were available for testing, and they showed inconsistent DCT results (Table 1). The results of the VIM-1-producing strain may be due to its 3-fold lower catalytic efficiency compared with the VIM-2-producing strain [13]. Whether this slow reaction is typical of IMP-7, VIM-1, and VIM-3 remains questionable and requires further study. Two P. putida isolates with VIM-2-like MBLs showed positive reactions in 10-60 min depending on the repeat tests. The minimum inhibitory concentration of imipenem for all these five strains with slow DCT reactions was >32 µg/mL, suggesting that the slow positive reaction was not associated with the low level of resistance (data not shown).
The slightly faster positive reaction of Acinetobacter spp. with the VIM-2-like MBL (≤5 -30 min) compared with Pseudomonas spp. (≤5-50 min) with the same enzyme type may have been influenced by the larger amount of the usually thicker and non-sticky growth of Acinetobacter spp.
It was reported that all 24 isolates with OXA-48 and three with OXA-181 were Carba NP test positive [2]. Only two E. coli isolates with OXA-48 were DCT positive within 10 min, whereas the remaining 21 E. coli and K. pneumoniae isolates with OXA-232 were test negative, suggesting that the positive DCT is related to the higher imipenem catalytic activity (kcat/Km) of OXA-48 and OXA-181 than that of OXA-232 [14]. It was reported that A. baumannii isolates with OXA-23 and other OXA-type carbapenemases showed slow positive reactions to the Blue Carba test [5]. None of the 49 A. baumannii isolates studied, including 44 with an OXA-23-like gene, were DCT positive (Table 1), possibly due to the lower catalytic activity [15].
In our study, in all of the 51 randomly selected imipenem-non-susceptible isolates of Acinetobacter spp., both blaOXA-51-like- and blaOXA-23-like alleles were detected, indicating that the majority of these isolates currently in our institute are OXA-23-like enzyme-producing A. baumannii (data not shown). Likewise, all 46 carbapenem-resistant Acinetobacter spp. isolated from intensive care unit patients in China between 2012 and 2013 were blaOXA-23-like gene-positive A. baumannii, and MBL genes were not detected in any of the isolates [16].
In routine laboratory practice, laboratories that perform disk diffusion susceptibility testing can apply the DCT for carbapenem-non-susceptible isolates by using growth on agar plates. For laboratories performing the broth microdilution method, colonies grown on primary blood agar plates can be tested by sweep transfer to the DCT disks. Reliable positive results can be obtained even when using culture plates kept at 4℃ or at room temperature for a week. In fact, 2-day-old cultures tended to give more rapid positive reactions compared with cultures less than 24 hr of age (data not shown).
A limitation of the present study is that only one VIM-1-producing P. aeruginosa isolate was tested, and KPC-type carbapenemase-producing isolates were not present among the isolates tested for the DCT specificity. A second limitation is that all clinical isolates were only from Korea.
In conclusion, the DCT can be easily performed, even in small laboratories, for the rapid detection of isolates with KPC-type and NDM-type carbapenemases in addition to a majority of isolates with IMP-, VIM-, and SIM-type MBLs. The test is simple to perform, it does not require pH adjustment of the imipenem-cilastatin solution for disk preparation, and the prepared dried disks are stable at both -20℃ and 4℃. Cultures kept at 4℃ or room temperature can also be used, and a cell lysis procedure is not required. Lastly, the color development of the disk is observed at room temperature.
Acknowledgments
We thank Myung Sook Kim in the Section of Clinical Microbiology, Department of Laboratory Medicine, Yonsei University College of Medicine and Young Hee Seo in the Research Institute of Bacterial Resistance, Yonsei University College of Medicine for their invaluable help with this study.
References
1. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge test and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001; 7:88–91. PMID: 11298149.
2. Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012; 18:1503–1507. PMID: 22932472.
3. Dortet L, Poirel L, Nordmann P. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother. 2012; 56:6437–6440. PMID: 23070158.
4. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing.Twenty-fifth Informational supplement, M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute;2015.
5. Pires J, Novais A, Peixe L. Blue-carba, an easy biochemical test for detection of diverse carbapenemase producers directly from bacterial cultures. J Clin Microbiol. 2013; 51:4281–4283. PMID: 24108615.
6. Shiraki Y, Shibata N, Doi Y, Arakawa Y. Escherichia coli producing CTX-M-2 β-lactamase in cattle, Japan. Emerg Infect Dis. 2004; 10:69–75. PMID: 15078599.
7. Hong SS, Kim K, Huh JY, Jung B, Kang MS, Hong SG. Multiplex PCR for rapid detection of genes encoding class A carbapenemases. Ann Lab Med. 2012; 32:359–361. PMID: 22950072.
8. Solé M, Pitart C, Roca I, Fàbrega A, Salvador P, Muñoz L, et al. First description of an Escherichia coli strain producing NDM-1 carbapenemase in Spain. Antimicrob Agents Chemother. 2011; 55:4402–4404. PMID: 21730115.
9. Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, et al. Novel acquired metallo-β-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005; 49:4485–4491. PMID: 16251286.
10. Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006; 27:351–353. PMID: 16564159.
11. Tam VH, Chang KT, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, et al. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010; 54:1160–1164. PMID: 20086165.
12. Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013; 57:4578–4580. PMID: 23817380.
13. Docquier JD, Lamotte-Brasseur J, Galleni M, Amicosante G, Frère JM, Rossolini GM. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J Antimicrob Chemother. 2003; 51:257–266. PMID: 12562689.
14. Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, et al. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents. 2013; 41:325–329. PMID: 23305656.
15. Walther-Rasmussen J, Høiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006; 57:373–383. PMID: 16446375.
16. Zhou Y, Wu X, Zhang X, Hu Y, Yang X, Yang Z, et al. Genetic characterization of ST195 and ST365 carbapenem-resistant Acinetobacter baumannii harboring blaOXA-23 in Guangzhou, China. Microb Drug Resist. 2015; 21:386–390. PMID: 25602500.
Fig. 1
Examples of disk carbapenemase tests (A) and inhibition tests (B) showing the color of the disks at indicated times. Isolates were (1) Acinetobacter junii (AJU) 13/6/R1783; (2) Acinetobacter baumannii (ABA) 11/6/R2498; (3) A. baumannii 03/9/T104; (4) Acinetobacter nosocomialis (ANO) 15/4/B6548; (5) Pseudomonas aeruginosa (PAE) 11/11/U1203; (6) P. aeruginosa 97/143; (7) P. aeruginosa 95/1/704; (8) A. baumannii 15/1694; (9) Escherichia coli (ECO) 12/2/P57; (10) E. coli KD-1251; (11) Klebsiella pneumoniae (KPN) M0801, and (12) E. coli 15/5/141. Positive reactions progressed from greenish yellow to yellow. All KPC- and NDM-producing isolates showed fast and strong reactions, and the color progressed to orange yellow. An A. baumannii isolate with VIM-2 (2), a P. aeruginosa isolate with VIM-1 (6), and an E. coli isolate with OXA-48 (12) showed slow and weak reactions. A. baumannii with OXA-23 (8) did not show a positive reaction within 60 min. Panel B is an example of inhibition by 1,000 µg of TZ (or by 1,000 µg of TZ in PTZ) and 60 mM EDTA.
Abbreviations: PTZ, piperacillin-tazobactam; TZ, tazobactam.
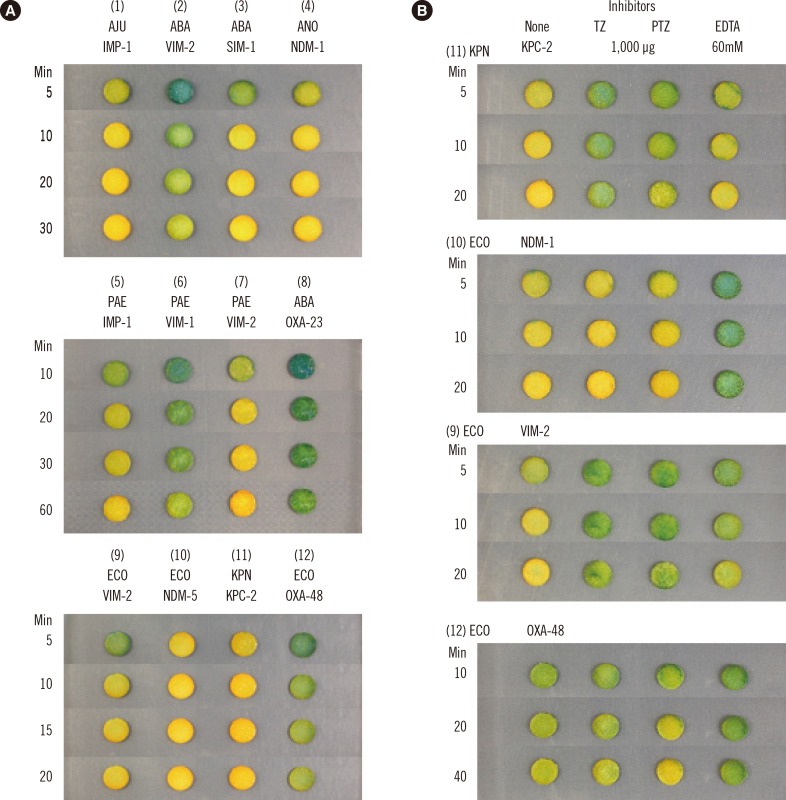
Table 1
Sensitivity of the disk carbapenemase test as determined by assessing known carbapenemase-producing reference strains and clinical isolates
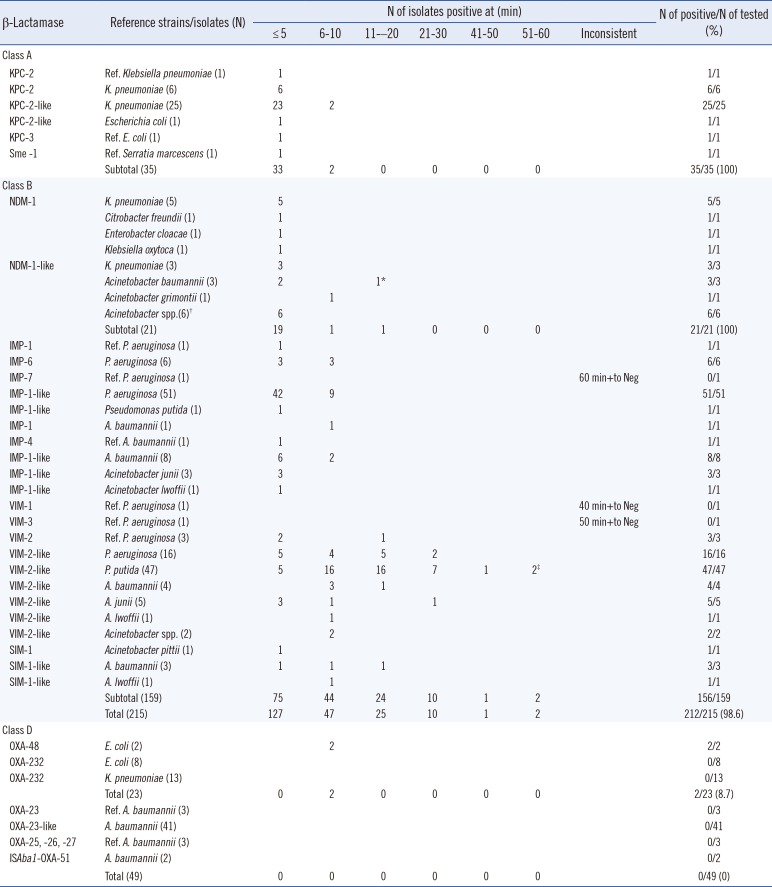
Table 2
Specificity of the disk carbapenemase test as determined by testing imipenem-nonsusceptible, but carbapenemase genes not tested isolates




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download