This article has been
cited by other articles in ScienceCentral.
Abstract
Background
We aimed to compare the diagnostic accuracy of the Alere Triage Cardio3 Tropinin I (TnI) assay (Alere, Inc., USA) and the PathFast cTnI-II (Mitsubishi Chemical Medience Corporation, Japan) against the central laboratory assay Singulex Erenna TnI assay (Singulex, USA).
Methods
Using the Markers in the Diagnosis of Acute Coronary Syndromes (MIDAS) study population, we evaluated the ability of three different assays to identify patients with acute myocardial infarction (AMI). The MIDAS dataset, described elsewhere, is a prospective multicenter dataset of emergency department (ED) patients with suspected acute coronary syndrome (ACS) and a planned objective myocardial perfusion evaluation. Myocardial infarction (MI) was diagnosed by central adjudication.
Results
The C-statistic with 95% confidence intervals (CI) for diagnosing MI by using a common population (n=241) was 0.95 (0.91-0.99), 0.95 (0.91-0.99), and 0.93 (0.89-0.97) for the Triage, Singulex, and PathFast assays, respectively. Of samples with detectable troponin, the absolute values had high Pearson (RP) and Spearman (RS) correlations and were RP =0.94 and RS=0.94 for Triage vs Singulex, RP =0.93 and RS=0.85 for Triage vs PathFast, and RP =0.89 and RS=0.73 for PathFast vs Singulex.
Conclusions
In a single comparative population of ED patients with suspected ACS, the Triage Cardio3 TnI, PathFast, and Singulex TnI assays provided similar diagnostic performance for MI.
Go to :

Keywords: Troponin, Point-of-care, Emergency medicine, Diagnostic accuracy
INTRODUCTION
Emergency medicine is practiced in a high pressure, high volume, time-sensitive clinical environment, where the value of rapid data acquisition is at a premium. Because early disposition decision-making in the emergency department (ED) can improve outcome and decrease mortality [
1234], processes that shorten the time for definitive diagnoses are of value. Point-of-care (POC) testing, defined as the performance of laboratory analyses in a near-patient location, eliminates many of the pre- and post-analytic sources of delay associated with performance in the central laboratory. In previous studies, POC testing decreased the time required to provide results to the physician by approximately 1 hr [
6], and showed a 2-hr reduction in the ED length of stay (ED LOS) in suspected myocardial infarction (MI) patients [
7]. However, the impact of POC testing, in terms of reduced time to results, must be considered in comparison to its potential negatives. While POC testing may yield results more rapidly than the laboratory-based assays, it has historically been generally at the cost of accuracy, compared to the results obtained by the central laboratory.
The purpose of this study was to compare the diagnostic accuracy of the Triage Cardio3 Troponin I (TnI) (Alere, Inc., San Diego, CA, USA) and the PathFast cTnI-II (Mitsubishi Chemical Medience Corporation, Tokyo, Japan) POC assays to the Singulex Erenna TnI assay (Alameda, CA, USA) laboratory-based platform, by using plasma samples from the Markers in the Diagnosis of Acute Coronary Syndromes (MIDAS) study.
Go to :

METHODS
MIDAS was a prospective, multi-center, blinded observational study that involved plasma samples from adult ED patients presenting with suspected acute coronary syndrome (ACS). It required that patients had symptoms for at least 10 min, with presentation and enrollment within 6 hr of symptom onset. MIDAS was registered at clinicaltrials.gov as #NCT01134913; the methods and description have been previously published [
8]. This study was sponsored by Alere, Inc., and approved by each participating institution's respective institutional review board. All participants provided informed consent. The authors had access to all data, and the manuscript was drafted by the first author.
Briefly, to be eligible for enrollment in MIDAS, the patient's presenting symptoms had to be such that the ED physician prospectively planned an objective evaluation for myocardial ischemia, including testing such as nuclear stress testing. Cardiac marker testing alone did not allow patient enrollment. Patient outcome for the endpoint MI vs no-MI was determined by three independent adjudicators unaffiliated with the MIDAS study sites. Adjudicators were provided clinical data summaries and completed case report forms, which were used to apply the criteria described in the 2012 publication ESC/ACCF/AHA/WHF Expert Consensus Document: Universal Definition of Myocardial Infarction [
9]. Unanimity among the adjudicators was required to assign a diagnosis of MI or no-MI. Adjudicators met to review the discrepant cases, and if this collective review determined that insufficient information was available to reach unanimity, a final diagnosis of "indeterminate" was assigned. Assays were evaluated for performance against the adjudicated outcomes.
Venous blood samples were collected in EDTA tube in the ED, during the period from 2006 to 2007. Plasma was aliquoted into cryotubes and then frozen at -80℃ within 1 hr of collection. Once frozen, the samples were shipped and analyzed by a core laboratory. While the Triage and PathFast assays were both tested using the same sample set, the enrollment measurements were not available for all patients; therefore, the actual number of analyzable samples differs slightly among Triage and PathFast assays.
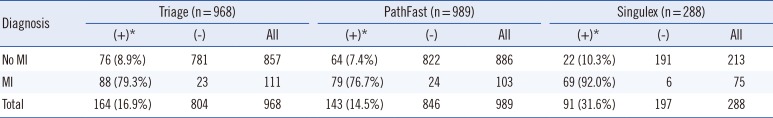
Table 1 lists the analyzable sample sizes. Because of the limitations in the available sample volume, the Singulex cohort was numerically smaller. Singulex assay results were measured in February 2011, and the Pathfast and Triage assay results were measured in September 2013.
Table 1
Gold standard diagnosis distribution for each TnI assay cohort
|
Diagnosis |
Triage (n=968) |
PathFast (n=989) |
Singulex (n=288) |
|
(+)*
|
(-) |
All |
(+)*
|
(-) |
All |
(+)*
|
(-) |
All |
|
No MI |
76 (8.9%) |
781 |
857 |
64 (7.4%) |
822 |
886 |
22 (10.3%) |
191 |
213 |
|
MI |
88 (79.3%) |
23 |
111 |
79 (76.7%) |
24 |
103 |
69 (92.0%) |
6 |
75 |
|
Total |
164 (16.9%) |
804 |
968 |
143 (14.5%) |
846 |
989 |
91 (31.6%) |
197 |
288 |

To ensure appropriate distribution of diagnoses in the Singulex cohort, we selected a predefined set of 295 samples, which were limited to the initial enrollment draw (0 hr), with a minimum of 720 µL of available plasma sample. Seven samples from the actual 301 patients were excluded because of the discrepancies between the sample bank volume estimates and the actual volume.
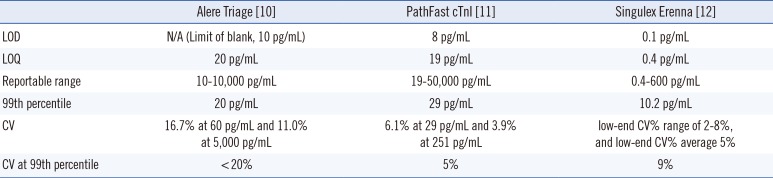
For the Triage Cardio3 TnI (Alere, Inc.) assay, the sample was treated with fluorescent antibody conjugates flowing through the test device by capillary action. The test device was inserted into the Triage Meter, and fluorescent signals are measured in approximately 20 min. Assay performance characteristics are reported in
Table 2 [
10]. The 99th-percentile of normal control subjects and the limit of quantitation (LOQ), defined as the lowest concentration with <20% CV, are both 20 pg/mL. The values below the lower limit were set to 10 pg/mL, while those above the upper limit were set to 10,000 pg/mL.
Table 2
Assay performance characteristics
|
Alere Triage [10] |
PathFast cTnI [11] |
Singulex Erenna [12] |
|
LOD |
N/A (Limit of blank, 10 pg/mL) |
8 pg/mL |
0.1 pg/mL |
|
LOQ |
20 pg/mL |
19 pg/mL |
0.4 pg/mL |
|
Reportable range |
10-10,000 pg/mL |
19-50,000 pg/mL |
0.4-600 pg/mL |
|
99th percentile |
20 pg/mL |
29 pg/mL |
10.2 pg/mL |
|
CV |
16.7% at 60 pg/mL and 11.0% at 5,000 pg/mL |
6.1% at 29 pg/mL and 3.9% at 251 pg/mL |
low-end CV% range of 2-8%, and low-end CV% average 5% |
|
CV at 99th percentile |
<20% |
5% |
9% |

The Mitsubishi PathFast cTnI-II test is a Food and Drug Administration (FDA)-approved chemiluminescent enzyme immunoassay for use in both clinical laboratory and POC settings. A sample is placed on the reagent cartridge and combined with antibody-coated magnetic particles and alkaline phosphatase conjugate. Troponin-bound complex emits photons, which are detected in the PathFast instrument and converted to troponin concentrations [
11]. Assay performance characteristics are reported in
Table 2.
Singulex Erenna TnI concentrations were measured in duplicate by using EDTA plasma specimens (Singulex, Alameda, CA, USA). The values below the lower LOQ were set to the lower LOQ, while those above the upper LOQ were set to the upper LOQ. The Singulex ZeptX System consists of flow immunoassays linked to a digital molecule-counting instrument. TnI-specific antibodies (BiosPacific, Emeryville, CA, USA) are used, and the sample is pumped through a glass capillary. Flow and volume are set such that a single fluorescent molecule passes through a laser beam at a time, with the fluorescence detected via an optical system coupled to a photon detector, thus providing high resolution of signal events [
11]. Assay performance characteristics are reported in
Table 2.
Statistical metrics were calculated by using SAS 9.3 (SAS Institute, Cary, NC, USA). Sensitivity, specificity, and predictive values were calculated at a clinical cutpoint defined as the 99th percentile of concentrations from a normal reference population [
13]. This value was 20 pg/mL for Triage TnI, 29 pg/mL for PathFast, and 10.2 pg/mL for Singulex test, based on the manufacturer's recommendations [
101112]. The degree of analytical correlation tested in samples with detectable troponin levels between the Triage, PathFast, and Singulex TnI tests was evaluated by using the Pearson and Spearman correlation statistic. Only subjects with measured values between the lower and upper limits for both assays in each comparison were included in correlation computations (Triage Cardio3: lower=10 pg/mL, upper=10,000 pg/mL; PathFast: lower=19 pg/mL, upper=50,000 pg/mL, and Singulex Erenna: lower=0.4 pg/mL, upper=600 pg/mL.).
Go to :

RESULTS
The total MIDAS cohort consisted of 1,018 patients of whom 557 (54.7%) were men, with a mean age of 58 (SD=13.4) yr, and a median time of presentation after symptom onset of 1.9 hr (interquartile rank [IQR]=1.1, 2.9). Overall, 681 (66.9%) patients were Caucasian, 265 (26.0%) patients were African American, 55 (5.4%) patients were Hispanic, and 12 (1.2%) patients were Asian. The initial creatinine level exceeded 1.5 mg/dL in 79 (7.8%) patients. At the index visit, 358 (35.2%) patients were discharged from the ED, with the remaining 660 (64.8%) being hospitalized. Comparison of the overall MIDAS population and the PathFast and Singulex cohorts showed no significant differences in any demographics or clinical characteristics.
Table 3 shows the statistical performance of all three assays, and
Fig. 1 shows the Bland Altman plots, and
Fig. 2, the Passing-Bablok Regression, comparing each of the assays.
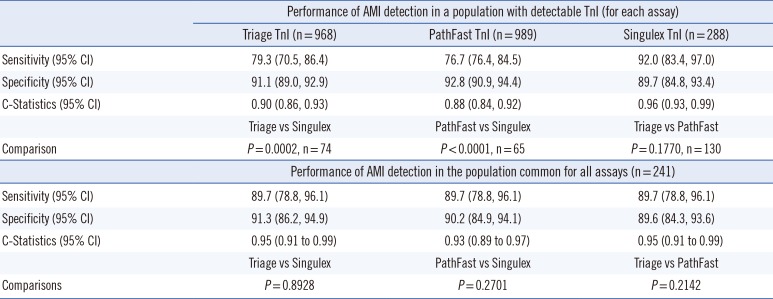
Table 3 presents the sensitivity and specificity for the three devices at the enrollment time point. In the first comparison of
Table 3, the cohort sizes vary because only those samples with detectable troponin levels were included, resulting in the following results; Triage vs PathFast (n=130), Triage vs Singulex (n=74), and PathFast vs Singulex (n=65). The C-statistic (95% confidence interval [CI]) for distinguishing MI from no-MI by using the enrollment draw was 0.90 (0.86, 0.93) for the Alere, 0.96 (0.93, 0.99) for the Singulex, and 0.88 (0.84, 0.92) for the PathFast assays. The
P values are 0.177 for Triage vs PathFast, 0.0002 for Triage vs Singulex, and <0.0001 for Pathfast vs Singulex. Enrollment draws with detectable troponin levels exhibited high-to-very-high correlation among the three assay platforms. Pearson (R
P) and Spearman (R
S) correlations, respectively, were R
P=0.94 and R
S=0.94 for Triage vs Singulex, R
P=0.93 and R
S=0.85 for Triage vs PathFast, and R
P=0.89 and R
S=0.73 for PathFast vs Singulex.
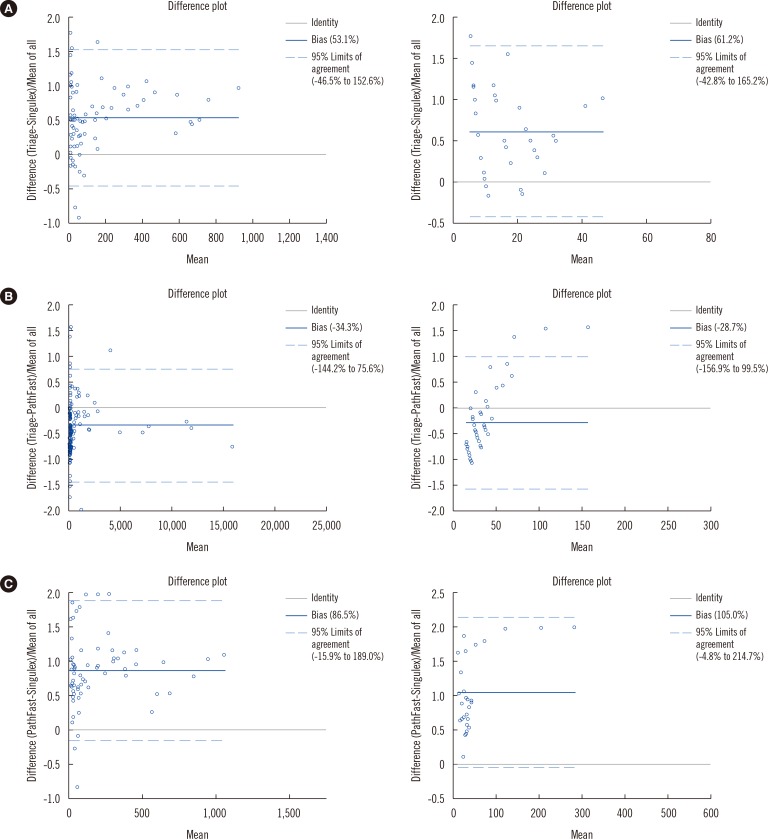 | Fig. 1Bland Altman plots of Triage TnI vs Singulex TnI (A), Triage TnI vs PathFast TnI (B), and PathFast TnI vs Singulex TnI (C). Samples above or below the measurable range for either assay were excluded.
|
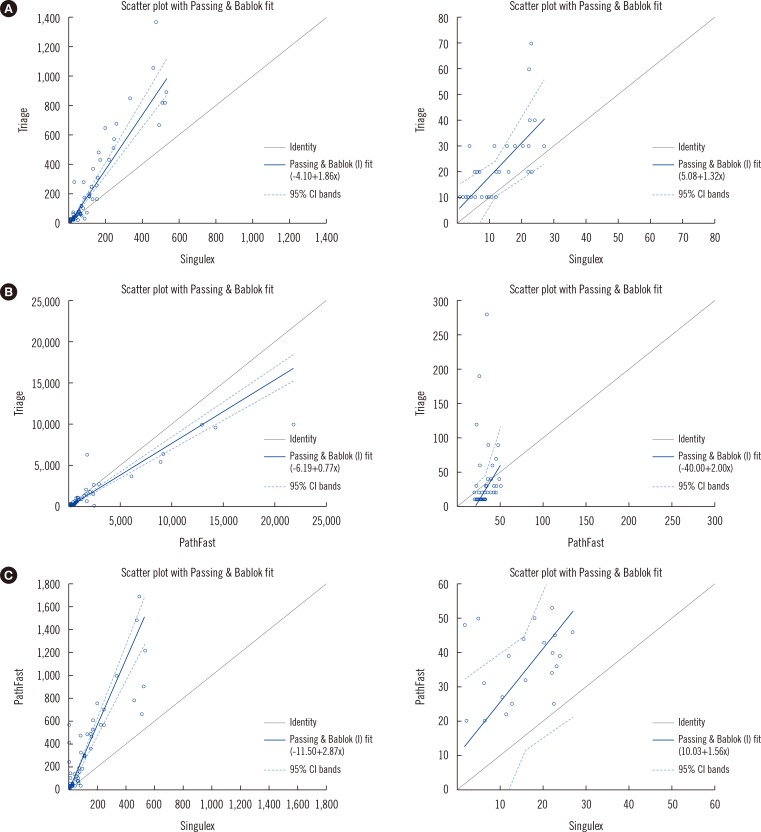 | Fig. 2
Passing-Bablok regression plots of Triage TnI vs Singulex TnI (A), Triage TnI vs PathFast TnI (B), and PathFast TnI vs Singulex TnI (C). Samples above or below the measurable range for either assay were excluded.
Abbreviation: CI, confidence interval.

|
Table 3
Sensitivity and specificity for AMI with 95% CI for each assay at the enrollment time point
|
Performance of AMI detection in a population with detectable TnI (for each assay) |
|
Triage TnI (n=968) |
PathFast TnI (n = 989) |
Singulex TnI (n = 288) |
|
Sensitivity (95% CI) |
79.3 (70.5, 86.4) |
76.7 (76.4, 84.5) |
92.0 (83.4, 97.0) |
|
Specificity (95% CI) |
91.1 (89.0, 92.9) |
92.8 (90.9, 94.4) |
89.7 (84.8, 93.4) |
|
C-Statistics (95% CI) |
0.90 (0.86, 0.93) |
0.88 (0.84, 0.92) |
0.96 (0.93, 0.99) |
|
Triage vs Singulex |
PathFast vs Singulex |
Triage vs PathFast |
|
Comparison |
P = 0.0002, n = 74 |
P < 0.0001, n = 65 |
P = 0.1770, n = 130 |
|
Performance of AMI detection in the population common for all assays (n = 241) |
|
Sensitivity (95% CI) |
89.7 (78.8, 96.1) |
89.7 (78.8, 96.1) |
89.7 (78.8, 96.1) |
|
Specificity (95% CI) |
91.3 (86.2, 94.9) |
90.2 (84.9, 94.1) |
89.6 (84.3, 93.6) |
|
C-Statistics (95% CI) |
0.95 (0.91 to 0.99) |
0.93 (0.89 to 0.97) |
0.95 (0.91 to 0.99) |
|
Triage vs Singulex |
PathFast vs Singulex |
Triage vs PathFast |
|
Comparisons |
P = 0.8928 |
P = 0.2701 |
P = 0.2142 |

The second part of
Table 3 shows the results obtained for 241 samples for which all three assays were performed and resulted in C-statistics (95% CI) for each assay's detection of acute myocardial infarction (AMI) of 0.95 (0.91-0.99), 0.93 (0.89-0.97), and 0.95 (0.91-0.99) for the Triage, PathFast, and Singulex assays, respectively.
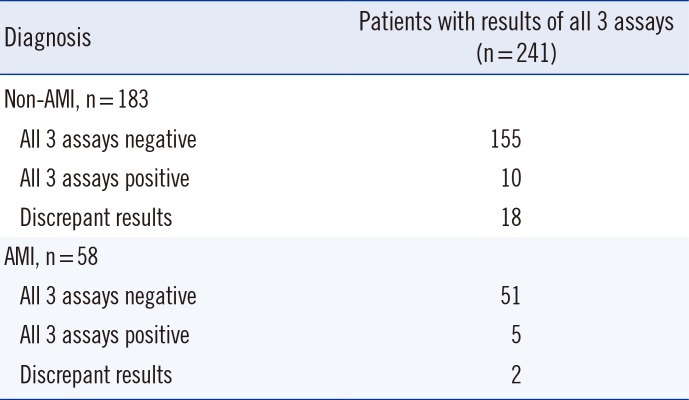
Table 4 shows the number of patients showing assay discrepancies.
Table 4
Details of patients with assay discrepancies
|
Diagnosis |
Patients with results of all 3 assays (n = 241) |
|
Non-AMI, n = 183 |
|
|
All 3 assays negative |
155 |
|
All 3 assays positive |
10 |
|
Discrepant results |
18 |
|
AMI, n = 58 |
|
|
All 3 assays negative |
51 |
|
All 3 assays positive |
5 |
|
Discrepant results |
2 |

Go to :

DISCUSSION
We have demonstrated that a contemporary POC assay can produce results that are statistically equivalent to the recently FDA-approved highly sensitive near-patient testing method PathFast TnI and the highly sensitive laboratory-based Singulex assay when tested in a common cohort. However, when tested individually by using the variable-sized enrollment population, the Singulex assay had a slightly higher C-statistic value that was statistically significant. While the Singulex assay has clearly demonstrated excellent performance as a research tool [
1314] and as a comparator in this study, the mechanics of its current performance, with a turn-around time measured in days, completely preclude its routine clinical application. Thus, with regard to the POC assays evaluated herein, if available for clinical use, these findings may provide practitioners greater opportunity to consider POC technology for clinical utility.
Each year, six million people [
16] present to the United States EDs with suspected ACS where decisions regarding their ultimate disposition depend on the troponin levels. While a troponin level exceeding the 99th percentile identifies a patient in whom additional evaluation is warranted, an overwhelming majority of patients presenting with suspected ACS undergo a lengthy work-up that is ultimately negative. Recent data suggest that rule-out protocols as short as 2 hr [
17] could greatly increase the speed to ED discharge in a subset of patients. Our results support the use of POC assays for shorter rule-out protocols.
Studies have shown that POC testing decreases the potential time-to-clinical decision-making by 1-2 hr [
67]. In today's busy EDs and intensive care units (ICUs), the rapid results obtained by POC testing have important implications. In fact, a recent report on >13 million ED visits pointed out that prolonged ED LOS (defined as >6 hr) is associated with 79% increase in acute mortality [
1]. Thus, decreasing the LOS by more rapid disposition decisions suggests that even the 1-hr advantage offered by POC testing has important implications. Conversely, beyond the level of disposition decision-making, some studies have suggested that an early invasive strategy (e.g., percutaneous coronary intervention) for selected non-ST segment elevation MI patients at high pre-test risk for adverse events may even improve mortality outcomes.
This study has several limitations. As a banked sample analysis performed in a central laboratory, it must be considered that the POC assay might possess different characteristics when the vagaries of the clinical environment are applied. Further, because the specimens used in this analysis were constructed from a sample bank, the clinical performance of the POC testing might be affected by the differences in the pre-test ACS probability when used in a different environment as well as ACS distribution. Moreover, because only the initial troponin test was selected, and because we used a patient population of ACS suspects, the diagnostic accuracy described in this study might not reflect the overall performance of these assays in clinical practice where serial measures are routinely performed.
It must also be considered that there is no concentration for which the Triage assay has a 10% CV. It should be pointed out that although guidelines recommend that the optimal CV at the 99th percentile upper reference limit be ≤10%, they do discuss that an assay at ≤20% is acceptable and has utility, while rejecting assays with a CV of >20% for clinical use. Ultimately, while the troponin values obtained rapidly from POC testing may allow earlier disposition, interventions, and greater transfer accuracy, the success of this approach depends on the non-rate-limiting nature of other processes.
We found that in a large population of suspected ACS patients, the Singulex assay provided the best statistical discrimination for the diagnosis of AMI. However, when a single common population was used for analysis, the Singulex, PathFast, and Triage assays were found to have similar diagnostic performance.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download