INTRODUCTION
Blood and blood components are essential for many patients. However, some patients may be exposed to risk factors during the administration of allogeneic blood or blood components [
1]. Adverse transfusion reactions (ATRs) can be infectious or non-infectious. The risk of infectious ATRs has dramatically decreased by approximately 10,000-fold because of strict donor screening and the development of techniques such as nucleic acid amplification tests and chemiluminescent immunoassays for detecting infectious agents [
2]. Besides, improved blood management systems have contributed to better quality of blood and blood components, and have decreased the risk associated with the transfusion procedure. The incidence of non-infectious ATRs has also decreased, but it still remains high [
3]. Although a majority of these reactions, such as febrile non-hemolytic transfusion reactions (FNHTRs) and allergic reactions are short-term and reversible, a few of these can cause significant morbidities, such as transfusion-related acute lung injury (TRALI) and transfusion- associated dyspnea (TAD), and mortality [
4]. Cytokines and antibodies that accumulate in stored allogeneic blood and blood components are responsible for these reactions [
3].
Many patients experience physical changes during transfusion. Most of these changes do not meet the criteria for ATRs or can be attributed to patients' underlying diseases and comorbidities [
1]. The overall incidence of ATRs is about 0.5-3% [
5], and types of ATRs vary according to pathophysiology, symptoms, and severity. The diagnosis of ATR may be difficult because of overlapping conditions in the patient and the underlying disease [
1]. Therefore, a comprehensive approach is required on part of the attending physician, such that ATR can be assumed once other differential diagnoses have been ruled out [
1,
6].
Until 2012, in our hospital, ATRs were directly reported by the clinicians in the transfusion wards, based on their clinical judgment. With no ATR report submitted to the blood bank, it was assumed that no ATR had occurred during the transfusion episode. However, this reporting process under-reported the incidence of ATRs, thus undermining the risk associated with the transfusion procedure. Notably, in order to comply with the requirements of the Joint Commission International (JCI) and the Korea Hemovigilance System (
http://www.kohevis.or.kr) on blood transfusion monitoring, our hospital introduced data-driven improvements in the ATR reporting process.
In this study, we measured the frequency and pattern of non-infectious ATRs at a tertiary care hospital using the newly developed online transfusion reaction reporting system and assessed the possible factors affecting these reactions with an aim to contribute to improved patient safety during transfusion.
Go to :

METHODS
1. Study design
Data for this study were collected from the electronic medical records (EMR) of Yonsei University Health System-affiliated Severance Hospital, a 2,089-bed tertiary care hospital in Seoul, Korea.
This study involved a retrospective observational analysis of two-year data (March 2013 to February 2015). Our institute has a computerized transfusion reaction reporting system integrated with the EMR. Whenever a patient is transfused, the concerned nurse is required to feed details about the ATRs in this system, irrespective of whether this information is mentioned in the nursing records. In case no ATR has occurred, the nurse is required to feed "no reaction" in the system. If one or more ATRs have occurred, the same are to be indicated in the electronic nursing records. In case of transfusion of red blood cells (RBCs), pre- and post-storage leukoreduced red blood cells (LR-RBCs), and single donor platelets (SDPs), a transfusion report is recorded for each blood unit transfused. Thus, the transfusion of one blood unit is regarded as one transfusion episode. In case of transfusion of whole blood-derived platelets (random donor platelets, RDPs), pooled leukoreduced platelets (LR-PLTs), fresh frozen plasmas (FFPs), and cryoprecipitates, the transfusion of six blood units is considered as one transfusion episode for calculating the incidence of ATRs.
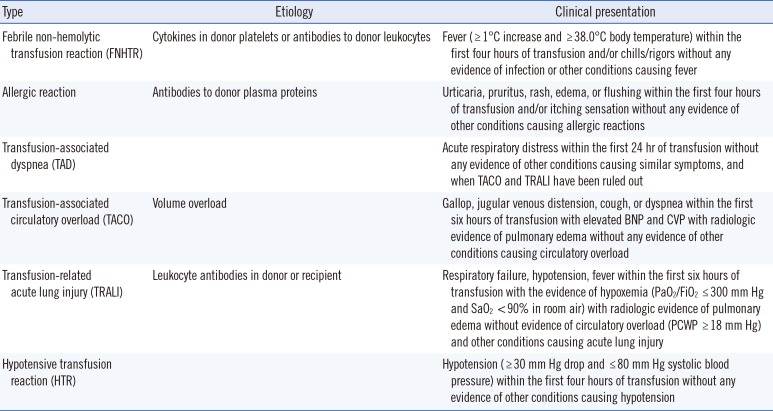
Different types of ATRs were defined in accordance with the criteria by the American Association of Blood Banks (AABB) [
6] and in the hemovigilance module surveillance protocol c.2.1.3 of the biovigilance component of the National Healthcare Safety Network of Centers for Disease Control and Prevention (CDC) [
7] (
Table 1). In our criteria, an acute hemolytic transfusion reaction (AHTR) was excluded because no AHTRs were reported due to our strict cross-matching and blood management system.
Table 1
Definition of different types of ATRs in accordance with the AABB [6] and CDC [7] criteria
|
Type |
Etiology |
Clinical presentation |
|
Febrile non-hemolytic transfusion reaction (FNHTR) |
Cytokines in donor platelets or antibodies to donor leukocytes |
Fever ( ≥ 1℃ increase and ≥ 38.0℃ body temperature) within the first four hours of transfusion and/or chills/rigors without any evidence of infection or other conditions causing fever |
|
Allergic reaction |
Antibodies to donor plasma proteins |
Urticaria, pruritus, rash, edema, or flushing within the first four hours of transfusion and/or itching sensation without any evidence of other conditions causing allergic reactions |
|
Transfusion-associated dyspnea (TAD) |
|
Acute respiratory distress within the first 24 hr of transfusion without any evidence of other conditions causing similar symptoms, and when TACO and TRALI have been ruled out |
|
Transfusion-associated circulatory overload (TACO) |
Volume overload |
Gallop, jugular venous distension, cough, or dyspnea within the first six hours of transfusion with elevated BNP and CVP with radiologic evidence of pulmonary edema without any evidence of other conditions causing circulatory overload |
|
Transfusion-related acute lung injury (TRALI) |
Leukocyte antibodies in donor or recipient |
Respiratory failure, hypotension, fever within the first six hours of transfusion with the evidence of hypoxemia (PaO2/FiO2 ≤ 300 mm Hg and SaO2 < 90% in room air) with radiologic evidence of pulmonary edema without evidence of circulatory overload (PCWP ≥ 18 mm Hg) and other conditions causing acute lung injury |
|
Hypotensive transfusion reaction (HTR) |
|
Hypotension ( ≥ 30 mm Hg drop and ≤ 80 mm Hg systolic blood pressure) within the first four hours of transfusion without any evidence of other conditions causing hypotension |

For this study, we initially collected data from the EMR on whether ATRs occurred. We also measured the proportion of ATRs reported in the nursing records. In case of ATRs, we collected information on the type of blood components transfused and the reported symptoms. Blood bank physicians confirmed and classified these cases of ATRs [
8] (
Fig. 1). Finally, these data were further analyzed according to the types of blood components transfused.
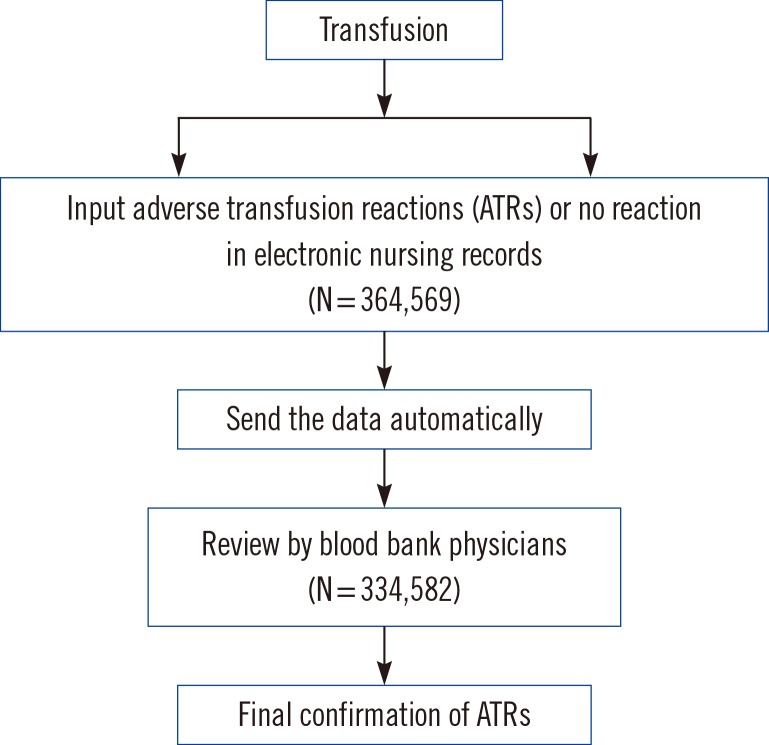 | Fig. 1Electronic audit system for monitoring adverse transfusion reactions.
|
2. Data collection
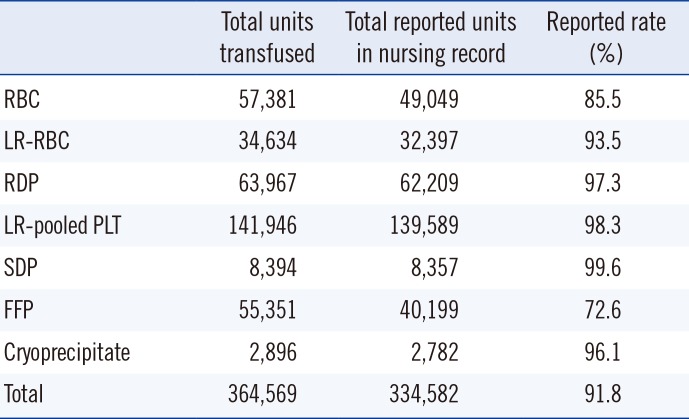
During the two years of the study, 364,569 units of blood were transfused. Of them, 334,582 (91.8%) records were identified in the nursing records. Transfusions were categorized as either ATR or no reaction. We summarized the reporting rate according to the blood components (
Table 2). The 8.2% data not reported in the nursing records involved transfusion in outpatients or in patients with transfusion during operation.
Table 2
Total transfusion units during March 2013 through February 2015 and reporting rate by nurses after 15 minutes of transfusion initiation
|
Total units transfused |
Total reported units in nursing record |
Reported rate (%) |
|
RBC |
57,381 |
49,049 |
85.5 |
|
LR-RBC |
34,634 |
32,397 |
93.5 |
|
RDP |
63,967 |
62,209 |
97.3 |
|
LR-pooled PLT |
141,946 |
139,589 |
98.3 |
|
SDP |
8,394 |
8,357 |
99.6 |
|
FFP |
55,351 |
40,199 |
72.6 |
|
Cryoprecipitate |
2,896 |
2,782 |
96.1 |
|
Total |
364,569 |
334,582 |
91.8 |

3. Statistical analysis
The frequencies of various symptoms and ATRs were calculated by dividing the number of cases associated with these symptoms and ATRs by the total number of transfusion episodes. The SPSS software (Version 18.0, Armonk, NY, USA) was used for statistical analysis. Statistical analyses involved the chi-square test and Fisher's exact test. P values below 0.05 were considered statistically significant.
Go to :

DISCUSSION
In our hospital, from March 2013 to February 2015, 91.8% of the units of blood components transfused were documented by nurses in the nursing records using a computerized transfusion reaction reporting system integrated with the EMR. In this study, the frequency of all possible transfusion-related events was 3.1% as per nursing records. However, after the blood bank physicians' review, this frequency was found to be 1.2%. The reported incidence of ATRs in the literature varied widely from 0.5% to 3% of all transfusion episodes [
5]. In our study, the frequency of fever, chills/rigors, and allergic reactions in the symptomatic cases were 53.1%, 10.6%, and 16.7%, respectively (
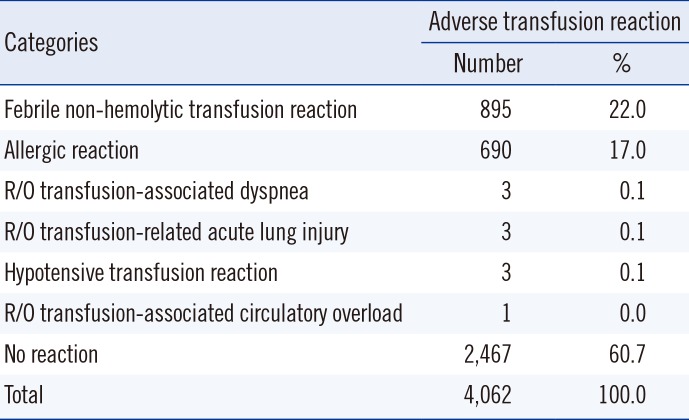
Table 3). However, the frequency of FNHTRs, allergic reactions, and "no reactions" determined by transfusion physicians among the symptomatic cases were 22.0%, 17.0%, and 60.7%, respectively (
Table 4). The discrepancy between the reported incidence by nurses and physicians was possibly because of the nurses' use of non-standardized criteria for defining various ATRs and overlooking the underlying disease conditions in the recipients. Markedly, three out of five reported clinical signs and symptoms did not meet the AABB [
6] and CDC criteria [
7]. Therefore, an adequate hemovigilance system needs to be established at the institutional, national, and international levels [
9].
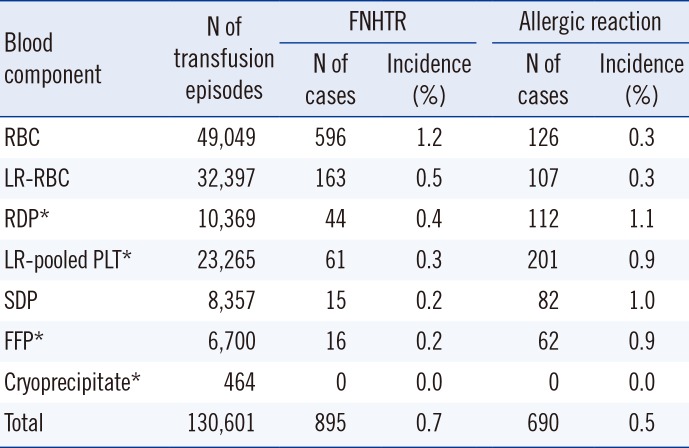
According to types of blood components, the frequency of FNHTRs to RBC components was significantly higher than those to FFPs and platelet components. In contrast, the frequency of allergic reactions to FFPs and platelet components was significantly higher than that to RBC components (
Table 5).
The previously reported incidence of FNHTRs to transfused RBC components ranged 0.25-0.5% [
3,
10,
11,
12]. In our study, it was 0.9%, a little higher than that reported in other studies. One possibility responsible for our higher rate may be that antipyretic premedications such as acetaminophen or diphenhydramine are not used in our hospital. Although recent studies found that premedication did not reduce the incidence of FNHTRs [
5], higher rates of fever and chills/rigors in our study may be attributed to the lack of premedication. Another possibility may be the less use of LR-RBCs in our setting compared with similar settings in other countries [
13]. The previously reported incidence of FNHTRs to transfused platelets ranged 0.22-0.33% [
3,
14,
15]. Similarly, the reported rate of 0.3% in our study falls within this range.
In case of allergic reactions, the previously reported incidence to transfused RBC components ranged 0.04-0.47% [
3,
10,
11,
12]. The reported rate of 0.3% in our study falls within this range. The previously reported rates of allergic reactions to transfused platelets ranged 0.09-2.82% [
3,
14,
15]. Again, the reported rate of 0.9% in our study falls within this range.
It is known that FNHTRs are the most common ATRs, which occur owing to the cytokines released from leukocytes during storage and the antigen-antibody reactions in leukocytes. This has led to a hypothesis that incidence of FNHTRs can be decreased through leukocyte reduction [
1,
16]. Many studies have been performed to verify this hypothesis. In line with various studies [
10,
11,
12,
14], we found that the use of LR-RBCs, LR-PLTs, and SDPs could decrease incidence of FNHTRs. However, in line with previous studies [
10,
11,
12,
14], as allergic reactions occur owing to antibodies against donor plasma proteins [
6,
7], the incidence of these reactions could not be significantly reduced by leukocyte reduction in RBCs and PLTs.
There were one case of suspected TACO and three cases of suspected TRALI. All patients immediately recovered with medication and short ventilation support. In Korea, TRALI cases are very rare because the country's population is ethnically homogenous and FFP from female donors has not been used for transfusion since 2009.
There are some limitations in this study. First, some of the transfusion records (approximately 8.2%) were not recorded by nurses. This reporting gap between the nurses and physicians could be addressed by framing more accurate input criteria with regard to ATRs. Besides, continuous education should be provided to nurses with special focus on monitoring cases of TACO and TRALI. Second, because our online transfusion reaction reporting system relied on the information from concerned nurses, an underreporting of delayed ATRs could occur. Although these delayed ATRs (such as delayed hemolytic transfusion reaction, delayed serologic transfusion reaction, transfusion-associated graft-versus-host disease, or post-transfusion purpura) are rare and difficult to identify, comprehensive education should be provided to nurses for identifying and reporting symptoms possibly related to delayed ATRs [
8].
We conducted a two-year retrospective study in a tertiary care hospital setting. We found that majority of the ATRs reported in this study were not serious. More significantly, we analyzed ATRs according to clinical correlates and blood components. Blood transfusion is a life-saving therapy; however, it also carries risks. Therefore, we need to closely monitor all transfusion episodes. By using the results of this study, prompt and effective provisions for preventing ATRs can be developed.
Before the implementation of the automatic reporting system, a few severe cases which require further problem-solving by blood bank physicians were reported. After the introduction of this system, we could count the overall transfusion reactions which developed in our hospital from mild to severe cases, and we could give good services with information of transfusion reactions to physicians and patients under the quality improvement of transfusion.
In conclusion, this type of reporting system may improve the estimation of incidences of ATRs, facilitate the accurate identification of underlying problems and risks, and contribute to quality transfusion care for patients.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download