This article has been
cited by other articles in ScienceCentral.
Abstract
The effects of storage temperatures, repeated freeze-thaw cycles, or delays in separating plasma or serum from blood samples are largely unknown for heat shock protein 27 (HSP27). We evaluated (1) the imprecision of the HSP27 assay used in this study; (2) the in vitro stability of HSP27 in blood samples stored at 4℃ for up to 6 hr with immediate and delayed serum/plasma separation from cells; and (3) the in vitro stability of HSP27 in blood samples stored at -80℃ after repeated freeze-thaw cycles. The ELISA to detect HSP27 in this study showed a within-run CV of <9% and a total CV of <15%. After 4-6 hr of storage at 4℃, HSP27 concentrations remained stable when using serum tubes irrespective of sample handling, but HSP27 concentrations decreased by 25-45% when using EDTA plasma tubes. Compared with baseline HSP27, one freeze-thaw cycle had no effect on serum concentrations. However, plasma concentrations increased by 3.1-fold after one freeze-thaw cycle and by 7.3-fold after five freeze-thaw cycles. In conclusion, serum is an appropriate biological sample type for use in epidemiological and large-scale clinical studies.
Go to :

Keywords: Heat shock proteins, In vitro stability, Storage conditions
Heat shock protein 27 (HSP27) is a member of the small heat shock protein family and has a molecular weight of approximately 27 kDa. HSP27 is thought to play an important role in many different conditions such as cancer and cardiovascular disease [
123]. Although an increasing number of studies have measured HSP27 concentrations in the serum or plasma [
456789101112], the effects of storage temperatures, repeated freeze-thaw cycles, or delays in separating plasma or serum are largely unknown.
In this study, we evaluated (1) the imprecision of the HSP27 assay used in this study; (2) the in vitro stability of HSP27 in blood samples stored at 4℃ for up to 6 hr with immediate and delayed serum/plasma separation from cells; and (3) the in vitro stability of HSP27 in blood samples stored at -80℃ after repeated freeze-thaw cycles.
1. HSP27 measurements
Blood samples were drawn from staff members of the Christian Doppler Laboratory for Cardiac and Thoracic Diagnosis and Regeneration at the Medical University of Vienna/Austria in autumn 2015. Informed consent was obtained from all healthy volunteers. Peripheral venous blood from participants was collected by venipuncture into K3 EDTA tubes and serum separator tubes (VACUETTE, Greiner Bio-One, Kremsmuenster, Austria).
Circulating HSP27 concentrations were measured by using the Human Total HSP27 DuoSet IC ELISA (Catalog No. DYC1580, R&D Systems, Minneapolis, MN, USA). This assay is designed to detect and quantify the concentration of HSP27 protein independent of its phosphorylation state. The measurement range of the R&D HSP27 assay is 31.20-2,000 ng/L when using undiluted samples. We determined HSP27 concentrations according to the manufacturer's instructions, but rather than using undiluted samples, we used a sample dilution of 1:4 to account for the HSP27 blood concentrations typically observed in previously published studies [
713]. HSP27 concentrations of each serum/plasma sample were measured in duplicate on the same micro-well plates. The mean of these two values was used for further analyses.
Go to :

2. Precision study
We evaluated the imprecision of the R&D HSP27 assay applying a protocol described by the CLSI guideline EP5-A [
14]. We used three pooled patient serum samples, which we had aliquoted into 20 plastic tubes for each concentration level and frozen at -80℃. We analyzed these samples in duplicate in one run per day for 20 days. Within-run and total CV was calculated by using the CLSI single-run precision evaluation test.
The R&D HSP27 assay showed a within-run CV of 8.8% and total CV of 12% at a mean serum concentration of 773 ng/L for pool 1, within-run CV of 6.6% and total CV of 12% at a mean serum concentration of 2,699 ng/L for pool 2, and within-run CV of 5.9% and total CV of 14% at a mean serum concentration of 4,509 ng/L for pool 3.
Thus, the imprecision data of the R&D HSP27 assay indicated a total CV of generally <15%, which is adequate for an ELISA format.
Go to :

3. In vitro stability of HSP27 in blood samples stored at 4℃
To evaluate the in vitro stability of HSP27 in blood samples stored at 4℃ for 2, 4, and 6 hr with immediate and delayed serum/plasma separation from cells, we collected serum and plasma samples from 10 healthy individuals. These samples were subsequently processed by using two different methods.
(1) Immediate sample processing: Following blood collection and after allowing a clotting time of 25 min for the serum tube, one serum and one plasma sample were subsequently fractioned by centrifugation (2,800g for 15 min) and then separated into four aliquots per sample type. One serum and one plasma aliquot were immediately analyzed to determine the baseline HSP27 concentrations. The remaining serum and plasma aliquots were stored at 4℃ for 2, 4, and 6 hr and used for HSP27 measurements after the respective time interval.
(2) Delayed sample processing: Following blood collection, blood samples in the serum and plasma tubes were stored at 4℃ for 2, 4, and 6 hr. After each storage period, one blood sample in the serum and plasma tubes per time point was fractioned by centrifugation (2,800g for 15 min), and then the obtained serum and plasma were used for HSP27 measurements.
The mean recovery of HSP27 was expressed as absolute values (i.e., absolute recovery) and calculated as the ratio of the value obtained after the given time interval of storage divided by the baseline value (i.e., relative recovery). For relative recovery, the default criterion for analyte stability was set at ±0.15; this implies that HSP27 was considered stable as long as the mean recovery differed by less than ±15% (a value derived from the total CV of the HSP27 assay used in this study).
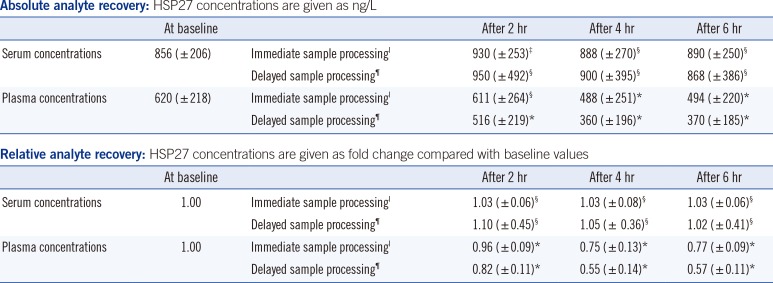
Table 1 summarizes the stability data of HSP27 after immediate and delayed processing of blood samples stored at 4℃. At baseline, we observed a matrix effect with higher mean serum than EDTA plasma concentrations (856 ng/L in serum vs. 620 ng/L in EDTA plasma). After 4-6 hr of storage, HSP27 concentrations remained stable when using serum tubes, regardless of the sample handling procedure; the difference was less than±10%. In contrast, HSP27 concentrations decreased significantly when using EDTA tubes; after immediate and delayed sample processing, the mean relative values after 4-6 hr of storage decreased by ~25% and ~45%, respectively.
Table 1
Stability results for HSP27: effects of immediate and delayed processing of blood samples with storage at 4℃
|
Absolute analyte recovery: HSP27 concentrations are given as ng/L |
|
At baseline |
|
After 2 hr |
After 4 hr |
After 6 hr |
|
Serum concentrations |
856 ( ± 206) |
Immediate sample processingI
|
930 ( ± 253)‡
|
888 ( ± 270)§
|
890 ( ± 250)§
|
|
Delayed sample processing¶
|
950 ( ± 492)§
|
900 ( ± 395)§
|
868 ( ± 386)§
|
|
Plasma concentrations |
620 ( ± 218) |
Immediate sample processingI
|
611 ( ± 264)§
|
488 ( ± 251)*
|
494 ( ± 220)*
|
|
Delayed sample processing¶
|
516 ( ± 219)*
|
360 ( ± 196)*
|
370 ( ± 185)*
|
|
Relative analyte recovery: HSP27 concentrations are given as fold change compared with baseline values |
|
At baseline |
|
After 2 hr |
After 4 hr |
After 6 hr |
|
Serum concentrations |
1.00 |
Immediate sample processingI
|
1.03 ( ± 0.06)§
|
1.03 ( ± 0.08)§
|
1.03 ( ± 0.06)§
|
|
Delayed sample processing¶
|
1.10 ( ± 0.45)§
|
1.05 ( ± 0.36)§
|
1.02 ( ± 0.41)§
|
|
Plasma concentrations |
1.00 |
Immediate sample processingI
|
0.96 ( ± 0.09)*
|
0.75 ( ± 0.13)*
|
0.77 ( ± 0.09)*
|
|
Delayed sample processing¶
|
0.82 ( ± 0.11)*
|
0.55 ( ± 0.14)*
|
0.57 ( ± 0.11)*
|

Since the HSP27 concentrations remained stable when using serum tubes stored at 4℃ for at least 6 hr and HSP27 concentrations decreased significantly under the same storage conditions in EDTA anticoagulated blood, serum is an appropriate sample type for HSP27 determination after short-term storage.
However, the reason for HSP27 instability in EDTA anticoagulated blood remains unclear. Distinct proteases may be able to degrade HSP27 in the presence of EDTA. One possibility is that HSP27 is cleaved by plasmin in EDTA plasma; similar effects have been shown
in vitro and
ex vivo in a previous study [
15].
Go to :

4. In vitro stability of HSP27 in blood samples stored at -80℃
To evaluate the in vitro stability of HSP27 in blood samples stored at -80℃ after repeated freeze-thaw cycles, we collected one serum and one plasma sample from 10 healthy individuals. Following blood collection, the serum and plasma samples were centrifuged at 2,800g for 15 min and then separated into three aliquots per sample type. One serum and one plasma sample from each of the 10 healthy individuals were immediately analyzed in one run. The remaining serum and plasma aliquots were stored at -80℃. One serum and one plasma aliquot were subjected to five freeze-thaw cycles within a period of seven days, while the other serum and plasma aliquots were thawed once after seven days. Subsequently, HSP27 concentrations were measured in the serum and plasma aliquots in one run for all study participants.
Even for this part of our study, the mean recovery of HSP27 was expressed as absolute recovery and relative recovery, and again, analyte stability was assumed if the mean recovery changed by less than ±15%.
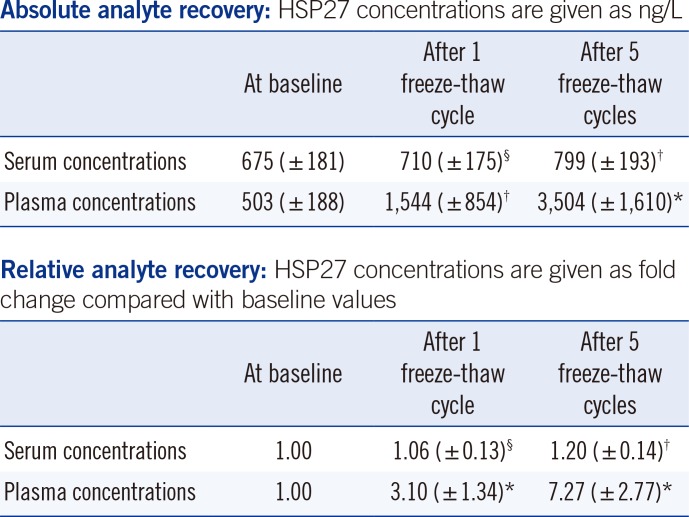
Compared with baseline HSP27 serum concentrations, one freeze-thaw cycle had no relevant effect on serum concentrations (i.e., 6% increase in the mean serum concentration after one freeze-thaw cycle). However, five freeze-thaw cycles resulted in 1.2-fold increased serum concentrations compared with the immediately analyzed samples. Notably, plasma concentrations increased by 3.1-fold after one freeze-thaw cycle and by 7.3-fold after five freeze-thaw cycles as shown in
Table 2 and
Fig. 1.
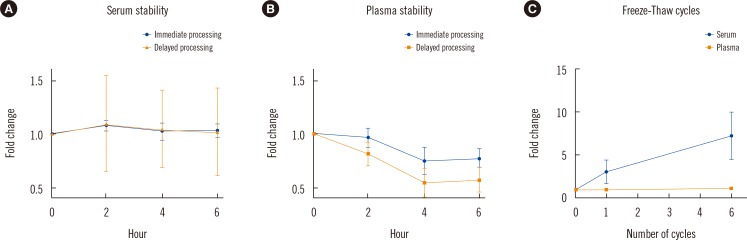 | Fig. 1
In vitro stability of HSP27 under different pre-analytical conditions: (A) relative analyte stability in serum samples and (B) plasma samples stored for 2-6 hr at 4℃ with immediate and delayed sample processing; and (C) the effect of repeated freeze-thaw cycles on HSP27 serum and plasma concentrations. Graphs show relative analyte recoveries at distinct time points (each dot represents the mean analyte concentrations relative to the baseline values of 10 healthy individuals; whiskers indicate standard deviation).
|
Table 2
Stability results for HSP27: effects of repeated freeze-thaw cycles with storage of serum/plasma samples at -80℃
|
Absolute analyte recovery: HSP27 concentrations are given as ng/L |
|
At baseline |
After 1
freeze-thaw cycle |
After 5
freeze-thaw cycles |
|
Serum concentrations |
675 (±181) |
710 (±175)§
|
799 (±193)†
|
|
Plasma concentrations |
503 (±188) |
1,544 (±854)†
|
3,504 (±1,610)*
|
|
Relative analyte recovery: HSP27 concentrations are given as fold change compared with baseline values |
|
At baseline |
After 1
freeze-thaw cycle |
After 5
freeze-thaw cycles |
|
Serum concentrations |
1.00 |
1.06 (±0.13)§
|
1.20 (±0.14)†
|
|
Plasma concentrations |
1.00 |
3.10 (±1.34)*
|
7.27 (±2.77)*
|

The significant increase in HSP27 plasma concentrations after one and five freeze-thaw cycles is clinically relevant. Thus, similar to our results for the analyte in vitro stability in blood samples stored at 4℃, it is not recommended to store EDTA plasma at -80℃. In contrast, serum appears to be an adequate sample type, but our results indicate that five freeze-thaw cycles are not acceptable in terms of analyte stability (>15% divergence). HSP27 concentrations decreased when the samples were stored at 4℃, whereas they increased when they were stored at -80℃ and thawed.
There may be several explanations for these observations. It was previously shown that HSP27 is phosphorylated at up to 10 positions and that HSP27 interacts with several proteins/structures (
http://www.uniprot.org/uniprot/P04792). Further, HSP27 is an oligomeric protein that is redistributed after being phosphorylated, forming tetramers and dimers [
15], but it remains unclear which form is present in the circulation. Thus, freezing and thawing of EDTA by itself or with coagulation factors might be able to alter the HSP27 structure, enabling the antibodies used in the assay to detect newly exposed epitopes. However, the results may also represent interference or non-specific binding. Further studies are necessary to clarify these issues.
Go to :

CONCLUSIONS
HSP27 concentrations remain stable for at least 6 hr in serum samples stored at 4℃ and after one freeze-thaw cycle in serum samples stored at -80℃. In contrast, the in vitro stability of HSP27 cannot be assumed in EDTA anticoagulated blood under both storage conditions. Thus, serum is an appropriate sample type for large-scale clinical trials.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download