Abstract
Background
Adverse transfusion reactions (ATRs) are clinically relevant to patients with significant morbidity and mortality. This study aimed to review the cases of ATR reported in the recipient-triggered trace back system for a recent nine-year period in Korea.
Methods
Nine-year data obtained from 2006 to 2014 by the trace back system at the Division of Human Blood Safety Surveillance of the Korean Centers for Disease Control (KCDC) were reviewed. The suspected cases were assessed according to six categories: (i) related to, (ii) probably related to, (iii) probably not related to, (iv) not related to transfusion, (v) unable to investigate, and (vi) under investigation.
Results
Since 2006, 199 suspected serious ATRs were reported in hospitals and medical institutions in Korea, and these ATRs were reassessed by the division of Human Blood Safety Surveillance of the KCDC. Among the reported 193 cases as transfusion related infections, hepatitis C virus (HCV) infection (135, 67.8%) was reported most frequently, followed by hepatitis B virus (HBV) infection (27, 13.6%), HIV infection (13, 6.5%), syphilis (9, 4.5%), malarial infection (4, 2.0%), other bacterial infections (3, 1.5%), HTLV infection (1, 0.5%), and scrub typhus infection (1, 0.5%), respectively. Of the 199 cases, 13 (6.5%) cases were confirmed as transfusion-related (3 HCV infections, 3 malarial infections, 1 HBV infection, 2 Staphylococcus aureus sepsis, 3 transfusion-related acute lung injuries, and 1 hemolytic transfusion reaction).
The national blood program was consigned to the Korean Red Cross (KRC) in 1981, but the emergence of six cases of transfusion-induced AIDS in 2004 instigated safety concerns from the public, and this led to strengthening the supervision of the national blood business to ensure blood safety [1]. The Korean blood transfusion community announced that they would initiate actions to further improve safety measures since September 2004, and the Korean Hemovigilance System has been started [2].There is no doubt that hemovigilance will continue to help improve the quality and safety of blood transfusions. Therefore, in many countries, hemovigilance networks have been introduced for monitoring adverse transfusion reactions (ATRs) and restructured according to the different health systems of the individual countries [3456]. Depending on social security and national priorities, various hemovigilance models have been used [78].
In Korea, ATRs in hospitals have been voluntarily reported to the Korean Hemovigilance System, whereas notifications of any ATR causing death, disability, hospitalization, or viral infection are mandatorily reported by the hospitals to the Korean Centers for Disease Control (KCDC). Initially, the KCDC took the lead in investigating ATRs; the division of Human Blood Safety Surveillance of the KCDC has monitored ATRs using a recipient trace back system since 2006. However, the reported individual cases have not been fully reviewed yet. Therefore, the purpose of this study was to analyze the nationwide cases of ATR reported from 2006 to 2014inan effort to improve the currently used Korean Hemovigilance System.
When suspected ATRs, including transfusion-transmitted infections (TTIs), are reported from medical institutions to community health centers, the centers forward the information to the division of Human Blood Safety Surveillance of the KCDC. These cases are further investigated by a system called the 'recipient-triggered trace back system'. Briefly, the division of Human Blood Safety Surveillance investigates the reported cases by: (i) collecting information about a recipient from the hospital; (ii) inquiring about the blood donation history of the donors by searching the Blood Information Sharing System at the KRC Blood Services; (iii) reviewing the medical records of donors and recipients with the aid of the Health Insurance Review & Assessment Service (HIRAS); (iv) repeat testing with archived blood samples of the corresponding blood donation; and (v) visiting blood donors for follow-up tests (Fig. 1).
The recipient-triggered trace back system was applied in cases of suspected ATR that were either detected following transfusion during the hospital treatment course or were brought up by outpatients themselves who had received transfusions in the past. Data collected by the trace back system from 2006 to 2014 were retrospectively reviewed and reassessed according to the following categories: (i) related to transfusion, (ii) probably related to transfusion, (iii) probably not related to transfusion, (iv) not related to transfusion, (v) unable to investigate, and (vi) under investigation. TTIs due to viruses were determined by serological tests for hepatitis B surface (HBs) antigen, HBs antibody, hepatitis B core (HBc) antibody, hepatitis C virus (HCV) antibody, HIV-1/2 antibody, human T cell leukemia virus (HTLV)-1 antibody, ALT, or a nucleic acid amplification test (NAT) for hepatitis B virus (HBV), HCV, and HIV. When malarial infection was suspected, malarial antibody test and PCR assay were performed on the stored samples obtained from donors. Bacterial TTI was demonstrated by paired blood cultures of patients and blood components. In addition to TTIs, transfusion-related acute lung injury (TRALI), acute hemolytic transfusion reaction (AHR), and the other adverse conditions were also investigated.
Of 199 reported cases, 12 (6.0%) could be confirmed as related to transfusion (Table 1 and Fig. 2). A half (49.2%) of the reported cases were categorized as unrelated to transfusion, 12 (6.0%) as probably not related to transfusion, 61 (30.7%) as unable to investigate, and 15 (7.5%) as under investigation.
Altogether, 199 serious adverse reactions were reported during the nine-year period from 2006 to 2014. HCV infection (135, 67.8%) was reported most frequently, followed by HBV infection (27, 13.6%), HIV infection (13, 6.5%), syphilis (9, 4.5%), malarial infection (4, 2.0%), and other bacterial infections (3, 1.5%)(Table 2).The annual reported numbers were as follows: 53 cases in 2006, 29 cases in 2007, 18 cases in 2008, 27 cases in 2009, 19 cases in 2010, 11 cases in 2011, 17 cases in 2012, 14 cases in 2013, and 11 cases in 2014.
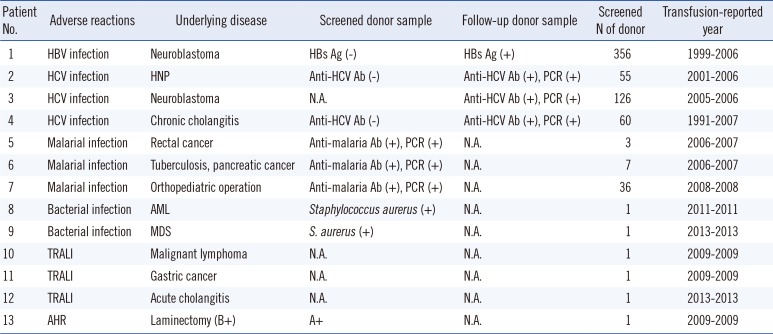
Thirteen of 199 reported cases were confirmed as 'related to transfusion' by the trace back system (Table 3). Thirteen confirmed cases were composed of nine cases of TTI, three cases of TRALI, and one case of AHR (Table 4). The TTI cases included three cases of HCV infection, three cases of malarial infection, one case of HBV infection, and two cases of Staphylococcus aureus sepsis.
The Joint United Kingdom Blood Transfusion Service Professional Advisory Committee has defined the "Trace Back" system as an investigation performed by the Blood Establishment when they receive a report that a patient has been identified with a TTI following the transfusion of blood components [9].This system was applied with modification to monitor severe ATRs in the division of Human Blood Safety Surveillance since 2006. Initially, this system mainly aimed at reducing TTIs, which have been problematic in Korea. Moreover, the Korean government encouraged reporting suspicious cases of TTI with a compensation fee paid when the cases were confirmed as 'category 1, related to transfusion'. This might be helpful to the development of the recipient trace back system, by detecting TTIs even in patients who were transfused over five years ago (Table 4).The gap between the transfusion year and report year could be explained by the claims of the recipients themselves. We found that the number of suspected cases (53 cases) encountered in the first year was 2-fold higher than that encountered in the other years, which might be due to the accumulation of data before the investigation. The suspected cases have been decreasing annually, and cases confirmed as TTI by HBV, HCV, and HIV has not been reported since the introduction of NATs.
The current study revealed only one case of HBV infection after transfusion in 1999. The donor was negative for HBs antigen in a donor screening test but was positive for HBs antigen after blood donation. This might be due to a relatively low analytical performance of the assay that could not overcome the window period of the donor's HBV infection at that time. In Korea, the residual risk for HBV was reduced from 2.18 patients to 1.47 patients per 0.1 million donors, after implementation of a chemiluminescence immunoassay (CLIA) (Abbott Laboratories, Diagnostic Division, Abbott Park, IL, USA) for HBs antigen [10]. However, the residual risk for HBV per million donations could differ from the prevalence in countries in which NATs were performed for donor screening: 2.8-3.6 in the United States, 1.4 in Britain, and 91.9 in southern Pakistan [111213].
HCV infection, accounting for 76.7% (135/176), was the most frequently reported viral TTI in this study. This frequency is higher than that determined by the 11-yr German hemovigilance system (58.7%, 1,699/2,894) [6]. Although the frequency of reported cases was high, the number of reports has markedly decreased after the introduction of strict donor selection with NAT, resulting in a minimized infectious window of 3.4 days after June 2012. Kim et al. [10] reported that the residual risks for HCV in Korea have declined 36.6-fold, from 1 in 81 thousand to 1 in 3 million, after the introduction of the NAT for HCV RNA. We found three cases of transfusion-related HCV infection, but all of them were transfused based on enzyme immunoassay (EIA) screening with a 58.3-day infectious window period before the introduction of the NAT. Considering that the most common cases reported as suspiciously transfusion related were HCV infections, the NAT might be helpful for promoting safety in transfusion.
Since the re-emergence of malaria in 1993, Plasmodium vivax has become endemic in Korea and is associated with military service along the demilitarized zone (DMZ) [14]. Seo et al. [15] reported that 60% of soldiers who served in the DMZ and were diagnosed with malaria in 1997 had donated their blood before diagnosis. In Korea, donor deferral criteria for cellular components (red blood cell and platelet) have been applied with restriction for periods of 12 or 36 months after domestic travel to, or residence in, malaria risk areas [16]. However, the current study found that three transfusion-mediated malaria cases occurred outside of malaria risk areas. Previous reports in the USA and UK supported that transfusion-transmitted malaria might be unpreventable solely by strict adherence to donor exclusion guidelines [17]. The World Health Organization recommends that all blood donations should be screened for malaria where "appropriate and possible", and that there should be quality-assured testing for TTIs [18]. However, these recommendations have significant resource implications. Inspection of malarial antigens or DNA in blood donations from malaria risk areas may be an additional solution for preventing transfusion-transmitted malaria.
It is noteworthy that this is the first Korean report of transfusion-transmitted bacterial sepsis caused by S. aureus described in an English-language journal, although there was a transfusion accident that 10 patients were dead in 1974 because of contaminated Serratia marcescens in glass blood bank bottles [19].Two cases were observed in patients who suffered from hematological malignancy and depended on repeated platelet transfusion. S. aureus was found in blood cultures of both recipients and in the platelet component. A definite route of contamination could not be determined, but the same characteristic, methicillin susceptibility, was demonstrated in S. aureus in both samples by an antimicrobial susceptibility test and molecular epidemiological tests, and bacterial TTIs were confirmed. Recently, the US Food and Drug Administration (FDA) announced that bacterial transmission during transfusion was the fourth most common cause of fatal transfusion-associated reactions in the United States [20]. Little is known about bacterial TTIs in Korea, whereas sepsis cases associated with platelet transfusion were reported in 1/25,000 transfusions in Canada and the United States [21]. As there are increasing numbers of immunocompromised patients with a tremendous need for blood transfusion, more attention should be paid to bacterial TTI. Prevalence of bacterial TTI might be underrecognized and underreported; thus, further efforts for creating a proper strategy in line with the Korean Hemovigilance System should be required, such as developing guidelines to clarify bacterial TTI cases in medical institutions or implementing proper screening tests for bacterial contamination of the platelet component.
In addition to TTIs, non-infectious transfusion reactions including three cases of TRALI and one case of hemolytic transfusion reaction were also investigated by this system. With respect to hemolytic transfusion reaction due to ABO-incompatible transfusion, 255 cases were reported over eight years in the UK by Serious Hazards of Transfusion (SHOT) and 226 cases over 10 yr in Japan [2223]. However, reported cases were limited in the Korean Hemovigilance System; only 12 cases were reported from April 2008 to November 2012. This might be the result of underreporting, considering that ABO-incompatible blood transfusion is likely to arise from human error [24]. However, systemic surveillance has not been performed owing to a lack of agreement within individual medical institutions for fear of penalties after reporting severe transfusion reactions. Only several cases of TRALI have been reported in Korea [25], although the US FDA has documented that the leading cause of transfusion-related fatality is TRALI [20]. Lack of awareness among healthcare professionals and lack of precise definitions might be the main reasons for this underreporting.
This is the first nationwide data concerning serious ATR cases for a recent nine-year period in Korea. Compared with data in the literature in the UK, USA, and Japan, many of the ATR cases might be still underreported, although the trace back system in Korea is mandatory. However, we expect that this study could contribute to improve the current Korean Hemovigilance System.
References
1. Cho JE. National blood management system and the direction of government policy in Korea. Korean J Hematol. 2010; 45:81–83. PMID: 21120182.

2. Kim S, Kim HO, Kim MJ, Lee SW, Shin YH, Choi YS, et al. Performance review of the National Blood Safety Improvement Project in Korea (2004-2009). Blood Res. 2013; 48:139–144. PMID: 23826584.

3. Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, et al. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002; 42:1356–1364. PMID: 12423521.

4. McClelland B, Love E, Scott S, Williamson LM. Haemovigilance: concept, Europe and UK initiatives. VoxSang. 1998; 74(Suppl 2):S431–S439.

5. Menitove JE. Hemovigilance in the United States of America. Vox Sang. 1998; 74(Suppl 2):S447–S455.

6. Keller-Stanislawski B, Lohmann A, Günay S, Heiden M, Funk MB. The German Haemovigilance System--reports of serious adverse transfusion reactions between 1997 and 2007. Transfus Med. 2009; 19:340–349. PMID: 19725904.

7. Otsubo H, Yamaguchi K. Current risks in blood transfusion in Japan. Jpn J Infect Dis. 2008; 61:427–433. PMID: 19050347.
8. Faber JC. Review of the main haemovigilance systems in the world. Transfus Clin Biol. 2009; 16:86–92. PMID: 19442556.
9. Joint United Kingdom Blood Transfusion and Tissue Transplantation Services (UKBTS) Professional Advisory Committee. Definition Look Back and Trace Back January 2015 Page 1 of 1. Updated on Jan 2015. http://www.transfusionguidelines.org.uk/document-library/general-documents.
10. Kim MJ, Park Q, Min HK, Kim HO. Residual risk of transfusion-transmitted infection with human immunodeficiency virus, hepatitis C virus, and hepatitis B virus in Korea from 2000 through 2010. BMC Infect Dis. 2012; 12:160. PMID: 22817275.

11. Zou S, Stramer SL, Notari EP, Kuhns MC, Krysztof D, Musavi F, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion. 2009; 49:1609–1620. PMID: 19413732.

12. Brant LJ, Reynolds C, Byrne L, Davison KL. Hepatitis B and residual risk of infection in English and Welsh blood donors, 1996 through 2008. Transfusion. 2011; 51:1493–1502. PMID: 21470235.

13. Moiz B, Moatter T, Shaikh U, Adil S, Ali N, Mahar F, et al. Estimating window period blood donations for human immunodeficiency virus Type 1, hepatitis C virus, and hepatitis B virus by nucleic acid amplification testing in Southern Pakistan. Transfusion. 2014; 54:1652–1659. PMID: 24383918.
14. Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the republic of Korea. Emerg Infect Dis. 1998; 4:295–297. PMID: 9621202.

15. Seo DH, Cho YH, Yeo WH, Hwang BK, Jung HJ, Hwang YS, et al. Analysis of blood donation history of Korean malaria patients. Korean J Clin Pathol. 1999; 19:569–571.
16. Korean Society of Blood Transfusion. The recommendation of standard blood bank and blood center service. 2nd ed. Seoul: Medrang Inforang;2007.
17. Purdy E, Perry E, Gorlin J, Jensen K. Transfusion-transmitted malaria: unpreventable by current donor exclusion guidelines? Transfusion. 2004; 44:464. PMID: 14996210.

18. World Health Organization. World malaria report 2014. Geneva: WHO;2014.
19. Cho HI, Seo DH, Kim HO. History of the Korean society of blood transfusion and blood services in Korea. Korean J Blood Transfus. 2012; 23:97–106.
20. US Food and Drug Administration. Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year 2012. Washington DC: US Food and Drug Administration;2014.
21. Eder AF, Kennedy JM, Dy BA, Notari EP, Weiss JW, Fang CT, et al. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004-2006). Transfusion. 2007; 47:1134–1142. PMID: 17581147.

22. Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006; 20:273–282. PMID: 17008165.

23. Fujii Y, Shibata Y, Miyata S, Inaba S, Asai T, Hoshi Y, et al. Consecutive national surveys of ABO-incompatible blood transfusion in Japan. Vox Sang. 2009; 97:240–246. PMID: 19476605.

24. Myhre BA, McRuer D. Human error-a significant cause of transfusion mortality. Transfusion. 2000; 40:879–885. PMID: 10924620.

25. Jin SM, Jang MJ, Huh JY, Park MH, Song EY, Oh D. A case of transfusion-related acute lung injury induced by anti-human leukocyte antigen antibodies in acute leukemia. Korean J Hematol. 2012; 47:302–306. PMID: 23320011.

Fig. 1
Flowchart of trace back system for the report and investigation of transfusion-related adverse reactions in Korean Hemovigilance System.
Abbreviation: KCDC, Korean Centers for Disease Control.
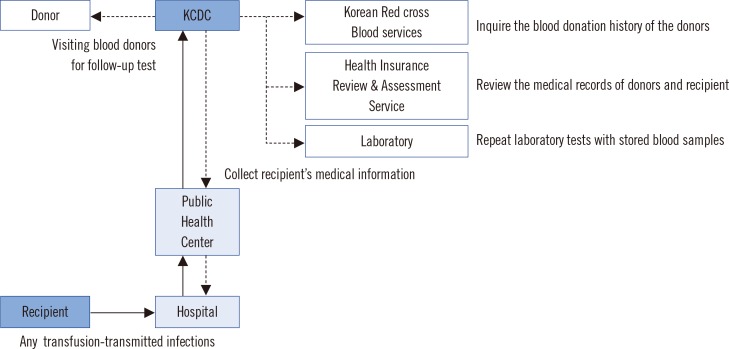
Fig. 2
Annual reported cases of suspected adverse reactions by the trace back system in Korea, 2006-2014.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HTLV, human T cell leukemia virus.
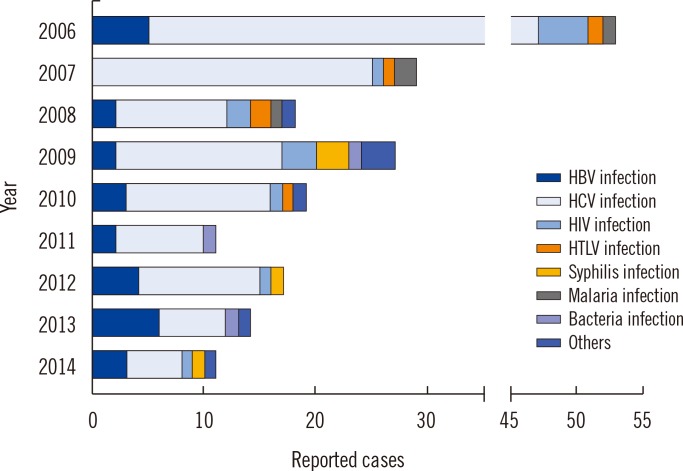
Table 1
Classification of the suspected adverse reaction cases investigated by the trace back system in Korea, 2006-2014
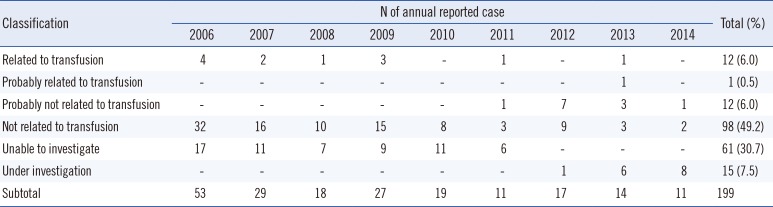
Table 2
Annual reported cases of suspected adverse reactions investigated by the recipient-triggered trace back in Korea, 2006-2014
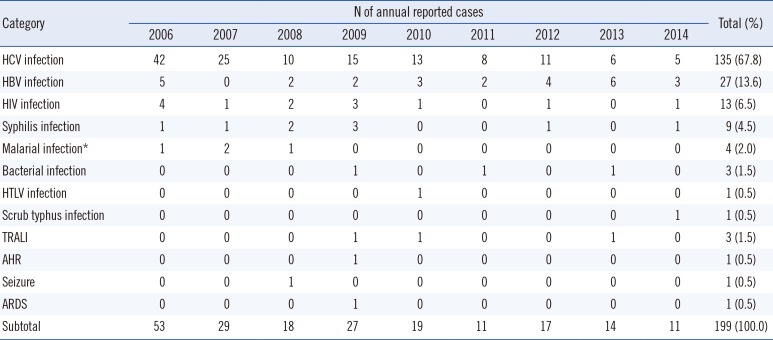
*Three cases of malaria were reported each year from 2006 to 2008 by Kim et al. [2].
Abbreviations: AHR, acute hemolytic transfusion reaction; ARDS, acute respiratory distress syndrome; HBV, hepatitis B virus; HCV, hepatitis C virus; HTLV, human T cell leukemia virus; TRALI, transfusion-related acute lung injury.
Table 3
Frequency of reported and confirmed transfusion-related adverse reactions (2006-2014)
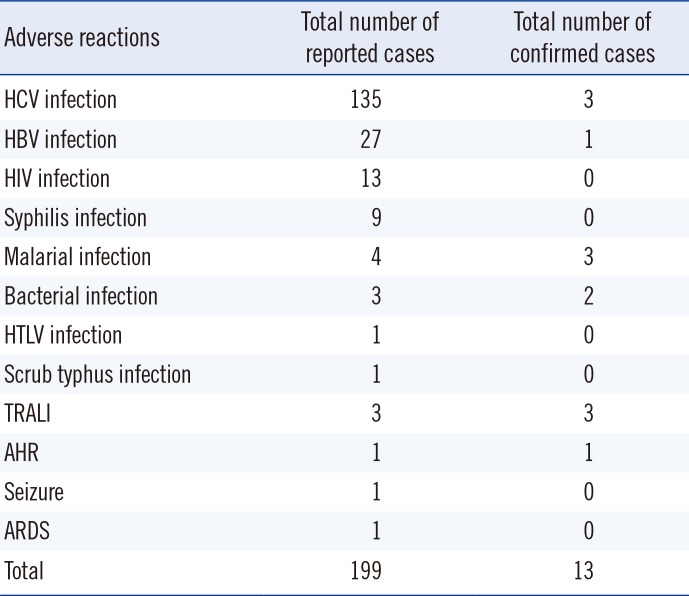
Table 4
Proven cases with adverse transfusion reaction investigated by the trace back system in Korea, 2006-2014




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download