Abstract
Background
Acinetobacter baumannii has a greater clinical impact and exhibits higher antimicrobial resistance rates than the non-baumannii Acinetobacter species. Therefore, the correct identification of Acinetobacter species is clinically important. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) has recently become the method of choice for identifying bacterial species. The purpose of this study was to evaluate the ability of MALDI-TOF MS (Bruker Daltonics GmbH, Germany) in combination with an improved database to identify various Acinetobacter species.
Methods
A total of 729 Acinetobacter clinical isolates were investigated, including 447 A. baumannii, 146 A. nosocomialis, 78 A. pittii, 18 A. ursingii, 9 A. bereziniae, 9 A. soli, 4 A. johnsonii, 4 A. radioresistens, 3 A. gyllenbergii, 3 A. haemolyticus, 2 A. lwoffii, 2 A. junii, 2 A. venetianus, and 2 A. genomospecies 14TU. After 212 isolates were tested with the default Bruker database, the profiles of 63 additional Acinetobacter strains were added to the default database, and 517 isolates from 32 hospitals were assayed for validation. All strains in this study were confirmed by rpoB sequencing.
Results
The addition of the 63 Acinetobacter strains' profiles to the default Bruker database increased the overall concordance rate between MALDI-TOF MS and rpoB sequencing from 69.8% (148/212) to 100.0% (517/517). Moreover, after library modification, all previously mismatched 64 Acinetobacter strains were correctly identified.
The Acinetobacter genus is composed of over 40 named species and nine genomic species [12]. Among these Acinetobacter species, Acinetobacter baumannii is the most clinically important pathogen because it is involved in nosocomial infections and often exhibits multidrug resistance, particularly to carbapenems [345]. Non-baumannii Acinetobacter species have been isolated from patients with bacteremia, endocarditis, and meningitis [678]. The clinical features and antibiotic susceptibilities of A. baumannii are different from those of non-baumannii Acinetobacter species. A previous study on the clinical characteristics of Acinetobacter infections found that no cases of infection with non-baumannii Acinetobacter species aggravated to severe sepsis or septic shock [9]. The overall antimicrobial susceptibilities of non-baumannii Acinetobacter species have been much higher than those of A. baumannii. The median number of administered antibiotics was also lower in the non-baumannii group [91011]. Therefore, the accurate identification of Acinetobacter species is clinically significant.
The currently available phenotypic identification systems cannot precisely distinguish Acinetobacter species. Since Acinetobacter species are phenotypically very similar, non-baumannii Acinetobacter species are often erroneously identified as A. baumannii, resulting in overestimation of the prevalence of A. baumannii [1213]. In contrast, several genotypic methods, developed to differentiate Acinetobacter species, have been more effective [141516]. Among these methodologies, the 16S ribosomal RNA (rRNA) and RNA polymerase β-subunit (rpoB) gene sequencing approaches have been widely used. The most fairly accurate method for the identification of Acinetobacter species was suggested to be rpoB gene sequencing, because of the abundance of rpoB polymorphisms in these species [141617]. However, these molecular techniques are laborious, expensive, and time-consuming, making them unsuitable for routine identification of Acinetobacter species in clinical laboratories [2].
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) has been progressively applied for the identification of diverse microorganisms such as bacteria, yeasts, and even mycobacteria in clinical microbiology laboratories [1819]. MALDI-TOF MS yields unique mass spectral fingerprints of these microorganisms that can be compared with a database of established fingerprints, thus enabling accurate identification [18]. This method is simple, fast, and cost-effective, requiring only small amounts of samples [20]. Although several studies have attempted to adapt MALDI-TOF MS for the identification of Acinetobacter species, MALDI-TOF MS was insufficient for species-level identification of Acinetobacter because of limited databases [220].
Therefore, we generated an improved database and evaluated the ability of MALDI-TOF MS in conjunction with our new database to identify a broad range of clinical Acinetobacter species, based on the results of rpoB sequencing.
A total of 729 Acinetobacter clinical isolates collected between January 2012 and May 2015 at 32 university hospitals in Korea were included in this study. The isolates were composed of 447 A. baumannii, 146 A. nosocomialis, 78 A. pittii, 18 A. ursingii, 9 A. bereziniae, 9 A. soli, 4 A. johnsonii, 4 A. radioresistens, 3 A. gyllenbergii, 3 A. haemolyticus, 2 A. lwoffii, 2 A. junii, 2 A. venetianus, and 2 A. genomospecies 14TU. After 212 strains (28 A. baumannii, 110 A. nosocomialis, 55 A. pittii, 3 A. ursingii, 3 A. bereziniae, 3 A. soli, 2 A. johnsonii, 2 A. radioresistens, 1 A. gyllenbergii, 1 A. haemolyticus, 1 A. lwoffii, 1 A. junii, 1 A. venetianus, and 1 A. genomospecies 14TU) were tested with the default Bruker database, the profiles of 63 Acinetobacter strains (31 A. nosocomialis, 11 A. pittii, 6 A. bereziniae, 6 A. soli, 2 A. ursingii, 1 A. gyllenbergii, 1 A. haemolyticus, 2 A. junii, 1 A. venetianus, and 2 A. genomospecies 14TU) were added to the database. The updated 63 Acinetobacter strains are presented in Fig. 1. The rpoB sequence of each of the updated 63 Acinetobacter strains showed more than 99.0% identity with that of the closest species. Additionally, 517 Acinetobacter isolates (419 A. baumannii, 36 A. nosocomialis, 23 A. pittii, 15 A. ursingii, 6 A. bereziniae, 6 A. soli, 2 A. johnsonii, 2 A. radioresistens, 2 A. gyllenbergii, 2 A. haemolyticus, 1 A. lwoffii, 1 A. junii, 1 A. venetianus, and 1 A. genomospecies 14TU) were assayed for validation. These clinical isolates were deposited in the National Culture Collection for Pathogens, supervised by the Korean Centers for Disease Control and Prevention. All strains were stored at -80℃; well-isolated colonies were used for all experiments after growth on MacConkey agar for 12-24 hr at 37℃.
Species were identified by using the Vitek 2 system (bioMérieux Vitek Inc., Hazelwood, MO, USA) and/or 16S rRNA and rpoB sequencing [1416]. These sequences were compared against sequence databases by using a Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast) [20]. Additional PCR amplification of blaOXA-51 was conducted to confirm A. baumannii [21].
Samples were prepared as previously described [20]. A 1-µL sterile loop was used to transfer each colony into 300 µL of distilled water, after which 900 µL of absolute ethanol was added. The suspension was centrifuged at 12,000g for 2 min and the resultant supernatant was discarded. After the pellet was dried at room temperature, 50 µL of 70% formic acid (Sigma-Aldrich, St. Louis, MO, USA) was added. The mixture was vortexed vigorously to yield a homogeneous solution. Finally, 50 µL of acetonitrile (Sigma-Aldrich) was added, and the mixture was centrifuged at 12,000g for 2 min.
One microliter of each prepared bacterial extract was spotted onto a stainless steel target plate (Bruker Daltonics GmbH, Leipzig, Germany) and was dried at room temperature for 10 min. Next, 1 µL of matrix solution (consisting of alpha-cyano-4-hydroxycinnamic acid, absolute acetonitrile and trifluoroacetic acid) was placed onto the sample. The solution and sample were allowed to co-crystallize for 10 min at room temperature. The prepared samples were analyzed by using a MicroFlex LT mass spectrometer (Bruker Daltonics), which was operated using Flex control software (version 3.4; Bruker Daltonics). Calibration was conducted by using the Bacterial Test Standard (Bruker Daltonics) as recommended by the manufacturer.
Each prepared sample was analyzed on a MALDI Biotyper RTC (version 3.1; Bruker Daltonics); mass spectra were evaluated with Flex control software (Bruker Daltonics). Each mass spectrum was searched against the default Bruker database and against the updated database. The log score identification criteria, originating in the alignment of peaks to the best matching reference data, were classified as follows: ≥2.3, highly probable species; between 2.0 and 2.3, secure genus and probable species; between 1.7 and 2.0, probable genus; and <1.7, non-reliable identification. The dendrogram of the Acinetobacter isolates was constructed by using the correlation distance measurements and the average linkage algorithm settings of MALDI Biotyper3 (version 3.1; Bruker Daltonics).
Samples were prepared by using a Standard ethanol/formic acid extraction protocol as described earlier. One microliter of bacterial extract from each of the 63 Acinetobacter strains was spotted eight times onto a stainless steel target plate (Bruker Daltonics) and was dried for 10 min at room temperature. One microliter of matrix solution was also spotted onto each prepared sample. After drying at room temperature for 10 min, each spot was measured three times. The obtained 24 spectra were analyzed with Flex analysis software (version 3.4; Bruker Daltonics). Accurately determined mass spectra were uploaded into the MALDI Biotyper3 software (Bruker Daltonics) to create mean spectra (MSP) for Acinetobacter species according to the Biotyper MSP creation standard method.
The analyzed results with log score values are presented in Table 1. The species identification, which was confirmed by rpoB and 16S rRNA sequencing, has shown that MALDI-TOF MS yielded 69.8% (148/212) accuracy of identification at the species level with the default Bruker database and 100.0% (517/517) with the improved database for all of the studied isolates. When a log score of 2.0 was set as the cut-off value for probable species identification, the concordance rate between MALDI-TOF MS and sequencing increased from 72.4% (105/145) with the default Bruker database to 100.0% (514/514) with the improved database. Furthermore, if only the results of log score above 2.3 were included for reliable species identification, the concordance rate between MALDI-TOF MS and sequencing was elevated from 90.2% (55/61) with the default Bruker database to 100.0% (451/451) with the improved database.
Among the 64 discordant results for species identification with the default Bruker database, 49 A. nosocomialis and 5 A. pittii were erroneously identified as A. baumannii. The other 3 A. bereziniae, 3 A. soli, 1 A. gyllenbergii, 1 A. junii, 1 A. venetianus, and 1 A. genomospecies 14TU were misidentified as A. nosocomialis, A. baumannii, A. baylyi, A. gyllenbergii, or could not be identified by MALDI-TOF MS. In contrast, none of the 517 Acinetobacter strains for validation were misidentified when they were searched against the improved database using MALDI-TOF MS. Furthermore, the proportion of scores above 2.0 among the accurately identified isolates at the species level was 70.9% (105/148) using the default Bruker database, while the proportion was 99.4% when using improved database of MALDI-TOF MS (514/517). In particular, the proportion with log scores above 2.0 among the accurately identified A. nosocomialis increased from 50.8% (31/61) to 100.0% (36/36), and that of A. pittii increased from 76.0% (38/50) to 100.0% (23/23).
Representative mass spectra of 16 Acinetobacter species, generated by the Flex analysis (Bruker Daltonics), are presented in Fig. 2. In addition, a dendrogram was constructed by using 63 updated isolates from the improved database (Fig. 1). A. bereziniae, A. soli, A. gyllenbergii, A. junii, A. venetianus, and A. genomospecies 14TU, which were not included in the default Bruker database, are present in the dendrogram. A dendrogram, consisting of A. baumannii, A. nosocomialis, and A. pittii in the improved database, is shown in Fig. 3. Cluster analysis indicated that the protein signatures from A. baumannii species comprised a separate cluster, except those from A. baumannii CS 62_1 BRB, and were more closely related to those from A. nosocomialis than those from A. pittii.
MALDI-TOF MS has been effective in identifying a broad spectrum of microorganisms, including bacteria, yeasts, and molds [1819]. However, identification rates for Acinetobacter species have been suboptimal; only 72.4% (89/123) of studied Acinetobacter isolates were accurately identified [2]. In the present study, 72.4% (105/145) of Acinetobacter strains were validly identified at the species level if a log score of 2.0 was set as the cut-off value with the default Bruker database.
Most of the 64 discordant results consisted of 49 A. nosocomialis and 5 A. pittii, which were misidentified as A. baumannii by MALDI-TOF MS. Insufficient A. nosocomialis and A. pittii-specific protein signatures in the default Bruker database is one potential explanation for these identification failures. Kishii et al. [2] reported that the sensitivity improved from 74.8% to 82.4% after updating the default database with A. nosocomialis and A. pittii signatures. Additionally, 98.3% (59/60) of Acinetobacter isolates were identified at the species level, by using a local database that incorporated the specific signature profiles for A. nosocomialis into the default Bruker database, according to the process described by Espinal et al. [20]. In the present study, the overall concordance rate between MALDI-TOF MS and rpoB sequencing for the identification of Acinetobacter species was substantially increased from 69.8% (148/212) to 100.0% (517/517) after more diverse representative signatures were included within the default database.
Of particular note, all of the A. nosocomialis (36/36) and A. pittii (19/19) isolates were successfully identified after including a wide range of A. nosocomialis and A. pittii mass spectra into the default database. Moreover, database modification increased the proportion of scores above 2.0 among the correctly identified isolates from 70.9% (105/148) to 99.4% (514/517), further indicating the reliability of our results.
Three A. bereziniae, three A. soli, one A. gyllenbergii, one A. junii, one A. venetianus, and one A. genomospecies 14TU isolates were identified by MALDI-TOF MS after the incorporation of their protein signatures into the default Bruker database. These species could not previously be properly identified because of their absence in the default library. Kishii et al. [2] also correctly identified A. bereziniae and A. soli by MALDI-TOF MS, which had been misidentified as A. guillouiae and A. baylyi, after updating their original library.
Although the dendrogram showed that A. baumannii and A. nosocomialis were more closely related to each other than to other Acinetobacter species, these two species obviously formed separate clusters and MALDI-TOF MS analysis enabled the unambiguous identification of these two strains.
Peak analysis of representative Acinetobacter species yielded sufficient differences among their protein signatures for accurate MALDI-TOF MS-based identification. In the present study, the 5,749.1 m/z peak for A. baumannii; the 4,070.5 m/z and 8,139.0 m/z peaks for A. nosocomialis; and the 2,891.8 m/z, 4,412.6 m/z, and 5,760.0 m/z peaks for A. pittii were specific and similar to those reported in previous studies. Hsueh et al. [22] found that the 2,875.03 m/z and 5,749.51 m/z peaks were specific for A. baumannii; the 4,070.04 m/z and 8,137.58 m/z peaks were specific for A. nosocomialis; and the 2,889.87 m/z, 4,412.56 m/z, and 5,779.52 m/z peaks were specific for A. pittii. Šedo et al. [23] also reported that the 5,748 m/z peak was specific for A. baumannii.
The clinical relevance of non-baumannii Acinetobacter has been shown to differ substantially from that of A. baumannii [1]. Non-baumannii Acinetobacter species tend to be less prevalent than A. baumannii, although the prevalence of each Acinetobacter species may differ geographically. The majority of nosocomial Acinetobacter species isolated from patients during a 3-yr surveillance program were identified as A. baumannii (84.5%), whereas only 5.6% of infections were caused by A. pittii [24]. Turton et al. [12] also reported that most isolates were identified as A. baumannii (78%) among 690 Acinetobacter strains, whereas the incidences of A. lwoffii, A. ursingii, and A. pittii were 8.8%, 4%, and 1.7%, respectively. Our Acinetobacter isolates for validation consisted of 81.0% A. baumannii (419/517), 7.0% A. nosocomialis (36/517), and 4.4% A. pittii (23/517) strains, a finding that is consistent with previous results. The first group of 212 Acinetobacter species was mainly used for the verification of non-baumannii Acinetobacter species, which had been erroneously identified by MALDI-TOF MS. On the other hand, the second group, which consisted of 517 Acinetobacter species, was randomly collected specifically for the purpose of validating the improved database, and is therefore, a more descriptive of the prevalence of Acinetobacter species. The limitation of the present study was the use of two separate groups with differing strain distributions and numbers in evaluating the default and improved databases. A study analyzing the same groups with both the default and improved databases concurrently would provide a more accurate assessment of Acinetobacter prevalence.
With respect to clinical characteristics, A. baumannii has predominantly accounted for nosocomial infection outbreaks in intensive care units (ICU), although A. nosocomialis, A. pittii, and A. ursingii have also caused outbreaks [252627]. The rates of ICU admissions (15.4% vs. 50.0%), hospital-acquired infections (76.9% vs. 97.2%), and mortality (16.7% vs. 58.6%) were significantly greater in the A. baumannii group than in the non-A. baumannii group [911]. Moreover, Park et al. [28] found that A. baumannii infection was an independent predictor of mortality in patients with Acinetobacter bacteremia, a finding that further emphasizes the importance of correct species identification of Acinetobacter species.
Further, the antimicrobial susceptibilities of non-baumannii Acinetobacter species have been shown to differ greatly from those of A. baumannii. The resistance rates of A. nosocomialis and A. pittii to imipenem have been found to be 31% (4/13) and 6.7% (1/15), respectively, while that of A. baumannii was 66% (35/53) [29]. The overall rates of imipenem and/or meropenem resistance of the non-baumannii Acinetobacter isolates was 2.6% according to a previous report [14]. These differences likely originate from the different carbapenemases expressed by A. baumannii versus non-A. baumannii species [1]. Of particular interest, A. baumannii intrinsically harbors blaOXA-51, which can be used to differentiate A. baumannii from other Acinetobacter species. Additionally, Lee et al. [30] found that non-baumannii Acinetobacter isolates were more resistant to colistin than A. baumannii. Colistin has been widely used to treat A. baumannii infections; however, A. genomospecies 14TU is intrinsically resistant to colistin [31].
Taken together, accurate identification of Acinetobacter species is essential for appropriate management, which influences clinical outcomes. We demonstrated that refining the default MALDI-TOF MS database enabled the rapid and correct identification of Acinetobacter strains at the species level, thereby demonstrating the potential of this approach as an alternative to the use of laborious genotypic tools. In the present study, A. nosocomialis and A. pittii were the two most prevalent species after A. baumannii. These two species could be differentiated by MALDI-TOF MS by incorporating more diverse A. nosocomialis and A. pittii mass spectra. A. bereziniae, A. soli, A. gyllenbergii, A. junii, A. venetianus, and A. genomospecies 14TU were also identified after the default library was complemented with their missing protein signatures. Furthermore, to the best of our knowledge, this is the first demonstration of the correct identification of A. gyllenbergii, A. venetianus, and A. genomospecies 14TU clinical isolates by MALDI-TOF MS. Although this study could not include all of the Acinetobacter species nor a sufficient number of strains of rare Acinetobacter species, our data indicate that Acinetobacter species isolated in the clinical settings could be correctly identified. Moreover, the present study included a wide range of clinical isolates from various university hospitals, likely reflecting the diversity of species submitted to actual clinical laboratories.
In conclusion, our study showed that MALDI-TOF MS is an efficient technique for the accurate and rapid identification of Acinetobacter strains at the species level after library modification. The updated database, achieved by inclusion of more diverse and specific protein signatures for each Acinetobacter species into the default Bruker library, is required for correct species identification. The misidentification of clinical Acinetobacter isolates could lead to significant outcomes such as aggravation of infection or even death, given that the clinical relevance and antimicrobial susceptibilities of these species differ greatly. Therefore, MALDI-TOF MS has the potential to become an essential technique, by which clinical microbiology laboratories routinely identify Acinetobacter species.
Acknowledgements
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0756). Jin Nyoung Choi, and Bruker Daltonics Inc. provided technical support for data analysis. We also would like to thank the clinical microbiology laboratories of the 32 university hospitals in Korea for providing the various Acinetobacter strains in this study.
References
1. Espinal P, Roca I, Vila J. Clinical impact and molecular basis of antimicrobial resistance in non-baumannii Acinetobacter. Future Microbiol. 2011; 6:495–511. PMID: 21585259.

2. Kishii K, Kikuchi K, Matsuda N, Yoshida A, Okuzumi K, Uetera Y, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for species identification of Acinetobacter strains isolated from blood cultures. Clin Microbiol Infect. 2014; 20:424–430. PMID: 24125498.

3. Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, et al. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007; 45:3352–3359. PMID: 17715376.

4. Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012; 39:105–114. PMID: 22113193.

5. Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006; 42:692–699. PMID: 16447117.

6. Chen SF, Chang WN, Lu CH, Chuang YC, Tsai HH, Tsai NW, et al. Adult Acinetobacter meningitis and its comparison with non-Acinetobacter gram-negative bacterial meningitis. Acta Neurol Taiwan. 2005; 14:131–137. PMID: 16252615.
7. Tsai HY, Cheng A, Liu CY, Huang YT, Lee YC, Liao CH, et al. Bacteremia caused by Acinetobacter junii at a medical center in Taiwan, 2000-2010. Eur J Clin Microbiol Infect Dis. 2012; 31:2737–2743. PMID: 22562410.

8. Castellanos Martínez E, Telenti Asensio M, Rodríguez Blanco VM, Rodríguez Suárez ML, Morena Torrico A, Cortina Llosa A. Infective endocarditis of an interventricular patch caused by Acinetobacter haemolyticus. Infection. 1995; 23:243–245. PMID: 8522385.
9. Molina J, Cisneros JM, Fernández-Cuenca F, Rodríguez-Baño J, Ribera A, Beceiro A, et al. Clinical features of infections and colonization by Acinetobacter genospecies 3. J Clin Microbiol. 2010; 48:4623–4626. PMID: 20943868.
10. Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000; 31:690–697. PMID: 11017817.

11. Chuang YC, Sheng WH, Li SY, Lin YC, Wang JT, Chen YC, et al. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin Infect Dis. 2011; 52:352–360. PMID: 21193494.

12. Turton JF, Shah J, Ozongwu C, Pike R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J Clin Microbiol. 2010; 48:1445–1449. PMID: 20181894.
13. Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011; 52:879–891. PMID: 22028150.
14. Wang J, Ruan Z, Feng Y, Fu Y, Jiang Y, Wang H, et al. Species distribution of clinical Acinetobacter isolates revealed by different identification techniques. PLoS One. 2014; 9:e104882. PMID: 25120020.

15. Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, Chang TC. Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J Clin Microbiol. 2005; 43:1632–1639. PMID: 15814977.
16. Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology. 2009; 155:2333–2341. PMID: 19389786.

17. La Scola B, Gundi VA, Khamis A, Raoult D. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 2006; 44:827–832. PMID: 16517861.
18. Carbonnelle E, Mesquita C, Bille E, Day N, Dauphin B, Beretti JL, et al. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin Biochem. 2011; 44:104–109. PMID: 20620134.

19. Murray PR. What is new in clinical microbiology-microbial identification by MALDI-TOF mass spectrometry: a paper from the 2011 William Beaumont Hospital Symposium on molecular pathology. J Mol Diagn. 2012; 14:419–423. PMID: 22795961.
20. Espinal P, Seifert H, Dijkshoorn L, Vila J, Roca I. Rapid and accurate identification of genomic species from the Acinetobacter baumannii (Ab) group by MALDI-TOF MS. Clin Microbiol Infect. 2012; 18:1097–1103. PMID: 22085042.

21. Jeong S, Kim JO, Jeong SH, Bae IK, Song W. Evaluation of peptide nucleic acid-mediated multiplex real-time PCR kits for rapid detection of carbapenemase genes in gram-negative clinical isolates. J Microbiol Methods. 2015; 113:4–9. PMID: 25819308.

22. Hsueh PR, Kuo LC, Chang TC, Lee TF, Teng SH, Chuang YC, et al. Evaluation of the Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of blood isolates of Acinetobacter species. J Clin Microbiol. 2014; 52:3095–3100. PMID: 24899038.

23. Šedo O, Nemec A, Křížová L, Kačalová M, Zdráhal Z. Improvement of MALDI-TOF MS profiling for the differentiation of species within the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. Syst Appl Microbiol. 2013; 36:572–578. PMID: 24054697.

24. Donnarumma F, Sergi S, Indorato C, Mastromei G, Monnanni R, Nicoletti P, et al. Molecular characterization of acinetobacter isolates collected in intensive care units of six hospitals in Florence, Italy, during a 3-year surveillance program: a population structure analysis. J Clin Microbiol. 2010; 48:1297–1304. PMID: 20181903.
25. Máder K, Terhes G, Hajdú E, Urbán E, Sóki J, Magyar T, et al. Outbreak of septicaemic cases caused by Acinetobacter ursingii in a neonatal intensive care unit. Int J Med Microbiol. 2010; 300:338–340. PMID: 19931486.

26. Idzenga D, Schouten MA, van Zanten AR. Outbreak of Acinetobacter genomic species 3 in a Dutch intensive care unit. J Hosp Infect. 2006; 63:485–487. PMID: 16815591.

27. van Dessel H, Kamp-Hopmans TE, Fluit AC, Brisse S, de Smet AM, Dijkshoorn L, et al. Outbreak of a susceptible strain of Acinetobacter species 13 (sensu Tjernberg and Ursing) in an adult neurosurgical intensive care unit. J Hosp Infect. 2002; 51:89–95. PMID: 12090795.

28. Park KH, Shin JH, Lee SY, Kim SH, Jang MO, Kang SJ, et al. The clinical characteristics, carbapenem resistance, and outcome of Acinetobacter bacteremia according to genospecies. PLoS One. 2013; 8:e65026. PMID: 23755171.

29. Lin YC, Sheng WH, Chen YC, Chang SC, Hsia KC, Li SY. Differences in carbapenem resistance genes among Acinetobacterbaumannii, Acinetobacter genospecies 3 and Acinetobacter genospecies 13TU in Taiwan. Int J Antimicrob Agents. 2010; 35:439–443. PMID: 20106635.

30. Lee YT, Huang LY, Chiang DH, Chen CP, Chen TL, Wang FD, et al. Differences in phenotypic and genotypic characteristics among imipenem-non-susceptible Acinetobacter isolates belonging to different genomic species in Taiwan. Int J Antimicrob Agents. 2009; 34:580–584. PMID: 19733035.

31. Nemec A, Dijkshoorn L. Variations in colistin susceptibility among different species of the genus Acinetobacter. J Antimicrob Chemother. 2010; 65:367–369. PMID: 20008049.

Fig. 1
Dendrogram of the protein signatures of the updated 63 Acinetobacter strains, derived from matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) data. A. bereziniae, A. soli, A. gyllenbergii, A. junii, A. venetianus, and A. genomospecies 14TU that were not in the default Bruker database are presented. All distance values are relative and normalized to a maximal value of 1,000.

Fig. 2
Peak profiles of 15 representative Acinetobacter species generated by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). The mass-to-charge (m/z) ratios of the ions are shown on the x-axis, and the absolute intensities of the ions are presented on the y-axis. AU values were given by the software.
Abbreviation: AU, arbitrary intensity.
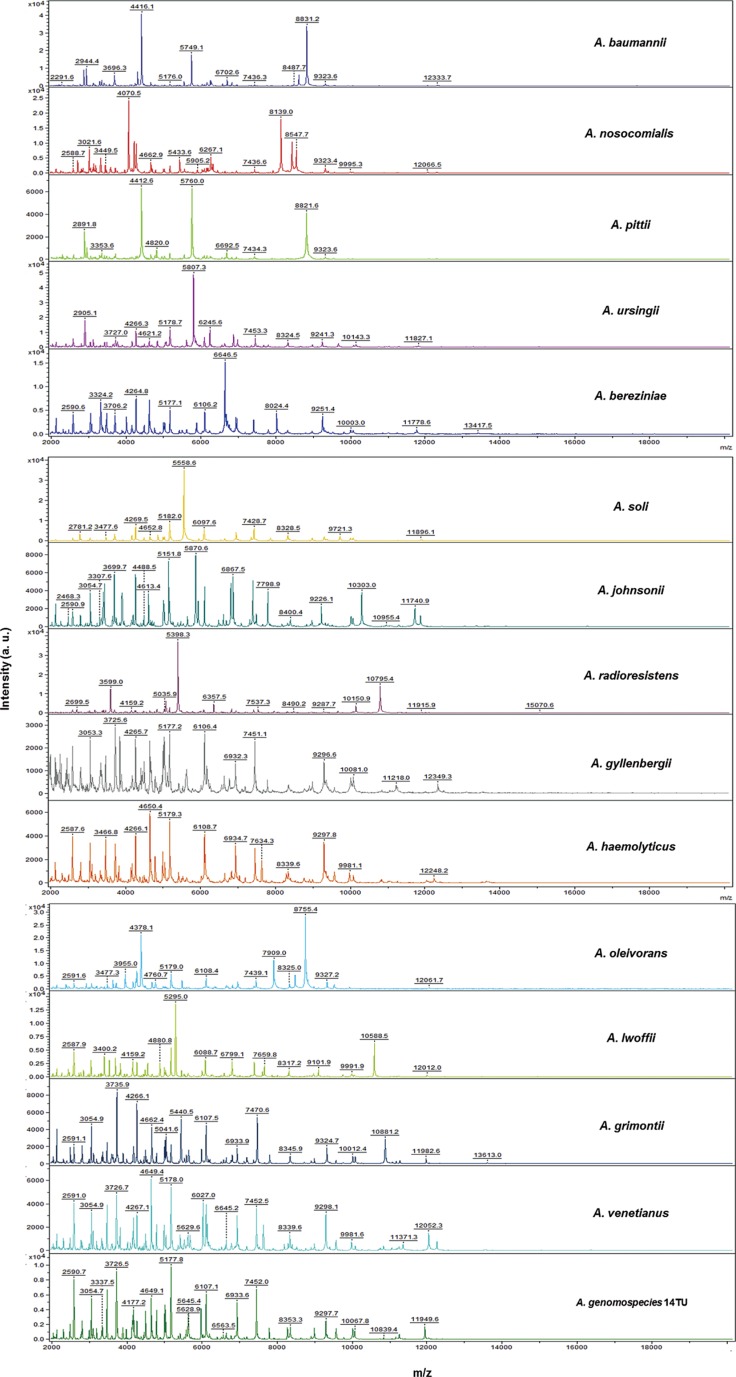
Fig. 3
Dendrogram constructed from the specific matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS)-generated mass spectra of A. baumannii, A. nosocomialis, and A. pittii within the improved database (which includes the original Bruker database). The protein profiles from A. baumannii isolates formed a separate cluster. The A. baumannii protein profiles were more closely associated with those from A. nosocomialis than those from A. pittii. All relative distance values are normalized to a maximal value of 1,000.
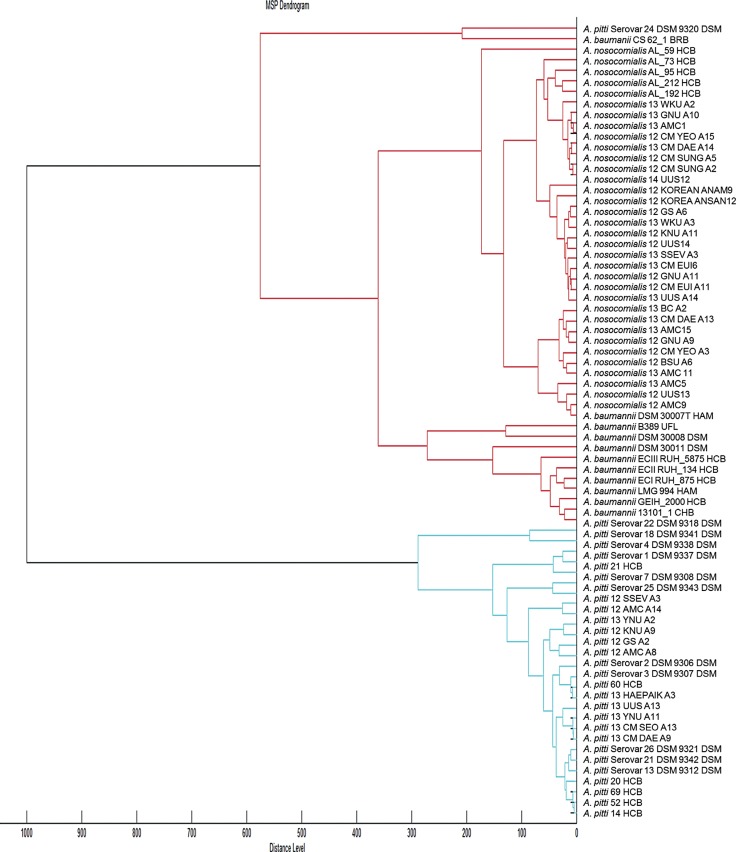
Table 1
Identification of Acinetobacter species and the associated log score values, obtained by using MALDI-TOF MS
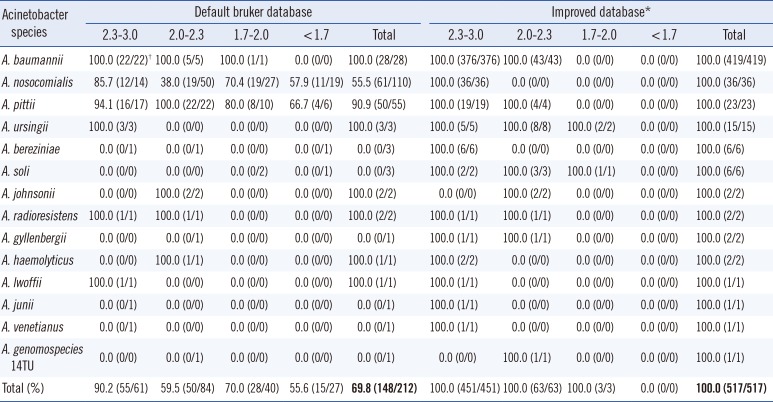
*The improved database consisted of the default Bruker database with the addition of representative mass spectra from 63 Acinetobacter strains; †(Number of concordant results between MALDI-TOF MS and rpoB gene sequencing/Number of all studied isolates confirmed by sequencing).
Abbreviations: MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download