This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Acute heart failure negatively affects short-term outcomes of patients with acute coronary syndrome (ACS). Therefore, reliable and non-invasive assessment of pulmonary congestion is needed to select patients requiring more intensive monitoring and therapy. Since plasma levels of natriuretic peptides are influenced by myocardial ischemia, they might not reliably reflect congestion in the context of ACS. The novel endothelial biomarker, soluble CD146 (sCD146), presents discriminative power for detecting the cardiac origin of acute dyspnea similar to that of natriuretic peptides and is associated with systemic congestion. We evaluated the performance of sCD146 for the assessment of pulmonary congestion in the early phase of ACS.
Methods
One thousand twenty-one consecutive patients with ACS were prospectively enrolled. Plasma levels of sCD146, brain natriuretic peptide (BNP), and high-sensitive troponin T were measured within 24 hr after the onset of chest pain. Pulmonary congestion on chest radiography was determined and classified in three groups according to the degree of congestion.
Results
Nine hundred twenty-seven patients with ACS were analyzed. Ninety-two (10%) patients showed signs of pulmonary edema on chest radiography. Plasma levels of sCD146 reflected the radiological severity of pulmonary congestion. Higher plasma levels of sCD146 were associated with the worse degree of pulmonary congestion. In contrast to BNP, sCD146 levels were not affected by the level of troponin T.
Conclusions
The novel endothelial biomarker, sCD146, correlates with radiological severity of pulmonary congestion in the early phase of ACS and, in contrast to BNP, is not affected by the amount of myocardial cell necrosis.
Go to :

Keywords: Acute coronary syndrome, Myocardial infarction, Pulmonary congestion, Pulmonary edema, sCD146
INTRODUCTION
Acute coronary syndrome (ACS) is a common precipitating factor of acute heart failure (AHF), and the presence of pulmonary congestion in patients with ACS negatively affects short term outcomes [
12]. Reliable and non-invasive assessment of pulmonary congestion is of importance to select patients in need of more intensive monitoring and therapy. The use of biomarkers, in particular natriuretic peptides, was advocated for this purpose. However, levels of circulating natriuretic peptides are influenced by the amount of myocardial ischemia and, therefore, may not accurately reflect pulmonary congestion in the context of ACS [
34].
It was recently shown that soluble CD146 (sCD146), an endothelial biomarker of systemic congestion, has a discriminative power to detect the cardiac origin of acute dyspnea similar to that of natriuretic peptides [
5]. The aim of this study was the evaluation of sCD146 for the assessment of the severity of pulmonary congestion in the early phase of ACS.
Go to :

METHODS
One thousand twenty-one consecutive patients who had ACS and were admitted to the coronary care unit (CCU) of the Cardiology Department of the University Hospital of Brno (Czech Republic) from July 2009 to November 2012 were enrolled. The diagnosis of ACS was based on appropriate symptoms in conjunction with consistent changes on electrocardiogram, i.e., ST-segment elevation or depression, new left bundle branch block, or negative T wave [
6]. Exclusion criteria were as follows: age >85 yr or estimated life expectancy due to non-cardiovascular reasons <12 months; known or newly diagnosed malignancy, inflammatory disease, or connective-tissue disease; distance from the place of residence to the hospital of >100 km; absence of coronary stenosis with reduction of the intraluminal diameter >50% on coronary angiography. Venous blood samples were drawn immediately on hospital admission before percutaneous coronary intervention (PCI) for standard biochemical and hematological analyses as well as sCD146 measurement. Troponin T and brain natriuretic peptide (BNP) were measured in blood drawn exactly 24 hr after the onset of chest pain. Samples were centrifuged within 10 min in a refrigerated centrifuge and stored at -80℃. Troponin T was analyzed with the high-sensitive assay (Roche Diagnostics, Basel, Switzerland; normal values <0.014 µg/L), BNP was assessed by using the AxSYM BNP-Microparticle Enzyme Immunoassay (Abbott Laboratories, Chicago, IL, USA; normal values <100 pg/mL), and sCD146 levels were determined by ELISA (CY-QUANT ELISA sCD146, Biocytex, Marseille, France; normal values <320 ng/mL [
7]). Pulmonary congestion was assessed by conventional chest radiography at admission. Images were evaluated by certified radiologists and classified in three groups: no or mild congestion, interstitial pulmonary edema, and alveolar pulmonary edema.
Written informed consent was obtained from all subjects before their participation in the study. The study was performed in observation of national laws and in accordance with the ethical standards of the Declaration of Helsinki, and was approved by the Ethics Committee of Faculty Hospital Brno (Brno, Czech Republic).
Values are expressed as median (interquartile range [IQR]) or as number (percentage), as appropriate. Three groups were compared with the Chi-square or the Kruskal-Wallis H-test, as appropriate. For statistically significant differences between the groups, subsequent pairwise comparisons were performed by using Dunn's procedure with Bonferroni correction of the P value for multiple comparisons. ROC curve was analyzed to assess diagnostic performances of biomarkers. The null hypothesis was rejected with an adjusted two-sided P<0.05. All analyses were performed by using IBM SPSS Statistics, Version 21.0. (IBM Corp, Armonk, NY, USA).
Go to :

RESULTS
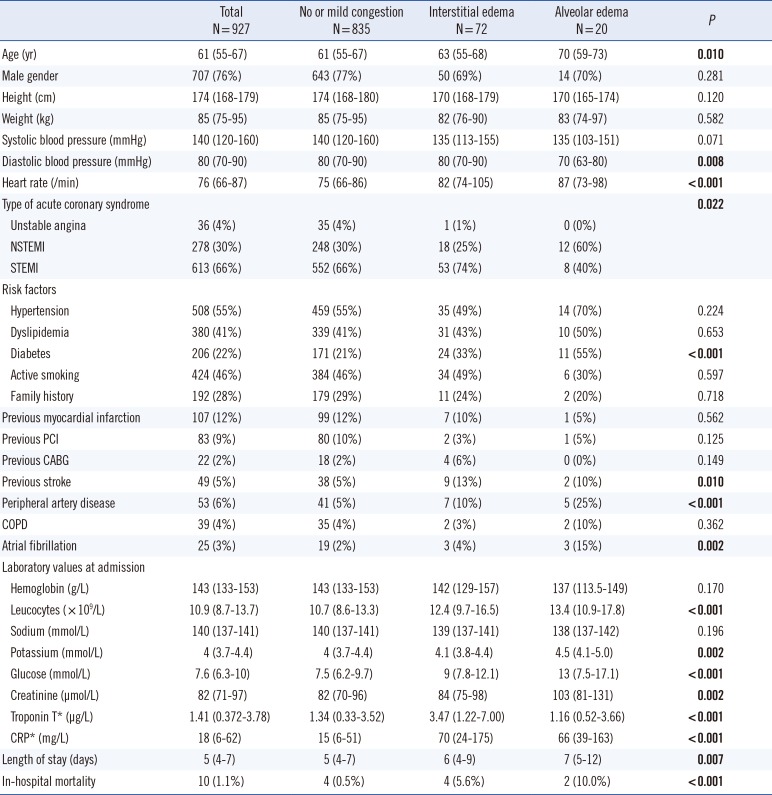
One thousand twenty-one patients presenting with ACS were prospectively screened. Patients without chest radiography at admission (n=94; 9%) were excluded from this analysis. Baseline characteristics of the 927 patients included in the study are summarized in
Table 1.
Table 1
Baseline characteristics of the patients
|
Total
N=927 |
No or mild congestion
N=835 |
Interstitial edema
N=72 |
Alveolar edema
N=20 |
P
|
|
Age (yr) |
61 (55-67) |
61 (55-67) |
63 (55-68) |
70 (59-73) |
0.010
|
|
Male gender |
707 (76%) |
643 (77%) |
50 (69%) |
14 (70%) |
0.281 |
|
Height (cm) |
174 (168-179) |
174 (168-180) |
170 (168-179) |
170 (165-174) |
0.120 |
|
Weight (kg) |
85 (75-95) |
85 (75-95) |
82 (76-90) |
83 (74-97) |
0.582 |
|
Systolic blood pressure (mmHg) |
140 (120-160) |
140 (120-160) |
135 (113-155) |
135 (103-151) |
0.071 |
|
Diastolic blood pressure (mmHg) |
80 (70-90) |
80 (70-90) |
80 (70-90) |
70 (63-80) |
0.008
|
|
Heart rate (/min) |
76 (66-87) |
75 (66-86) |
82 (74-105) |
87 (73-98) |
<0.001
|
|
Type of acute coronary syndrome |
|
|
|
|
0.022
|
|
Unstable angina |
36 (4%) |
35 (4%) |
1 (1%) |
0 (0%) |
|
NSTEMI |
278 (30%) |
248 (30%) |
18 (25%) |
12 (60%) |
|
STEMI |
613 (66%) |
552 (66%) |
53 (74%) |
8 (40%) |
|
Risk factors |
|
|
|
|
|
|
Hypertension |
508 (55%) |
459 (55%) |
35 (49%) |
14 (70%) |
0.224 |
|
Dyslipidemia |
380 (41%) |
339 (41%) |
31 (43%) |
10 (50%) |
0.653 |
|
Diabetes |
206 (22%) |
171 (21%) |
24 (33%) |
11 (55%) |
< 0.001
|
|
Active smoking |
424 (46%) |
384 (46%) |
34 (49%) |
6 (30%) |
0.597 |
|
Family history |
192 (28%) |
179 (29%) |
11 (24%) |
2 (20%) |
0.718 |
|
Previous myocardial infarction |
107 (12%) |
99 (12%) |
7 (10%) |
1 (5%) |
0.562 |
|
Previous PCI |
83 (9%) |
80 (10%) |
2 (3%) |
1 (5%) |
0.125 |
|
Previous CABG |
22 (2%) |
18 (2%) |
4 (6%) |
0 (0%) |
0.149 |
|
Previous stroke |
49 (5%) |
38 (5%) |
9 (13%) |
2 (10%) |
0.010
|
|
Peripheral artery disease |
53 (6%) |
41 (5%) |
7 (10%) |
5 (25%) |
< 0.001
|
|
COPD |
39 (4%) |
35 (4%) |
2 (3%) |
2 (10%) |
0.362 |
|
Atrial fibrillation |
25 (3%) |
19 (2%) |
3 (4%) |
3 (15%) |
0.002
|
|
Laboratory values at admission |
|
|
|
|
|
|
Hemoglobin (g/L) |
143 (133-153) |
143 (133-153) |
142 (129-157) |
137 (113.5-149) |
0.170 |
|
Leucocytes ( × 109/L) |
10.9 (8.7-13.7) |
10.7 (8.6-13.3) |
12.4 (9.7-16.5) |
13.4 (10.9-17.8) |
< 0.001
|
|
Sodium (mmol/L) |
140 (137-141) |
140 (137-141) |
139 (137-141) |
138 (137-142) |
0.196 |
|
Potassium (mmol/L) |
4 (3.7-4.4) |
4 (3.7-4.4) |
4.1 (3.8-4.4) |
4.5 (4.1-5.0) |
0.002
|
|
Glucose (mmol/L) |
7.6 (6.3-10) |
7.5 (6.2-9.7) |
9 (7.8-12.1) |
13 (7.5-17.1) |
< 0.001
|
|
Creatinine (µmol/L) |
82 (71-97) |
82 (70-96) |
84 (75-98) |
103 (81-131) |
0.002
|
|
Troponin T* (µg/L) |
1.41 (0.372-3.78) |
1.34 (0.33-3.52) |
3.47 (1.22-7.00) |
1.16 (0.52-3.66) |
< 0.001
|
|
CRP* (mg/L) |
18 (6-62) |
15 (6-51) |
70 (24-175) |
66 (39-163) |
< 0.001
|
|
Length of stay (days) |
5 (4-7) |
5 (4-7) |
6 (4-9) |
7 (5-12) |
0.007
|
|
In-hospital mortality |
10 (1.1%) |
4 (0.5%) |
4 (5.6%) |
2 (10.0%) |
< 0.001
|

Patients were subsequently classified in three groups according to the degree of pulmonary congestion on chest radiography at admission: no or mild congestion (n=835), interstitial edema (n=72), and alveolar edema (n=20).
Patients with severe pulmonary congestion presented more often with non-ST-elevation myocardial infarction (NSTEMI); were older; had higher prevalence of diabetes, atrial fibrillation, and peripheral artery disease; had higher creatinine levels; and lower diastolic blood pressure at admission compared with the other subgroups. Patients with no or mild congestion had lower heart rate, lower inflammation parameters, and lower glucose than the patients with severe congestion. Of note, interstitial edema was associated with higher troponin T levels than the other subgroups.
Patients with pulmonary congestion (interstitial or alveolar edema) presented an increased risk of in-hospital mortality compared with patients without or with mild pulmonary congestion (5.6% and 10.0% vs. 0.5%, P<0.001). There was also a trend toward longer hospital stay with increasing pulmonary congestion, although pairwise comparisons were not statistically significant.
For the overall population, the median level of sCD146 was 320 ng/mL (IQR 251-398 ng/mL, range 95-2,866 ng/mL) and the median level of BNP was 263 pg/mL (IQR 125-473 pg/mL, range 10-11,567 pg/mL).
Fig. 1 shows that median plasma levels of BNP were higher in patients with interstitial (679 pg/mL, IQR 355-1,097 pg/mL) or alveolar (665 pg/mL, IQR 267-1,214 pg/mL) pulmonary edema than the patients without or with mild signs of congestion (251 pg/mL, IQR 119-430 pg/mL). No difference between patients with interstitial and alveolar pulmonary edema was observed (
P values shown in
Fig. 1).
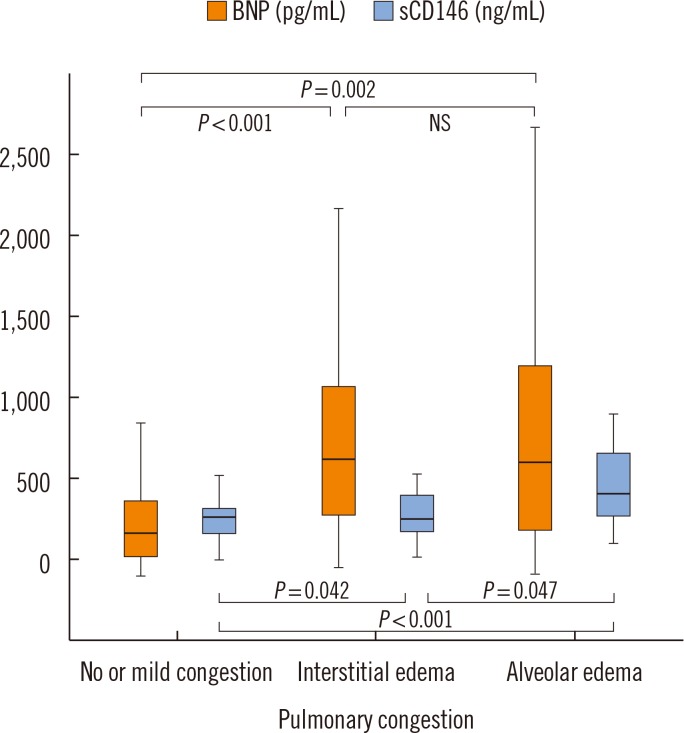 | Fig. 1
Plasma levels of BNP and sCD146 according to the severity of pulmonary congestion. Increasing radiological evidence of pulmonary congestion is associated with higher levels of sCD146. Plasma levels of BNP show higher dispersion and do not reflect the severity of pulmonary congestion. Median and interquartile range are displayed.
Abbreviations: BNP, brain natriuretic peptide; sCD146, soluble CD146; NS, not significant.

|
Fig. 1 further illustrates that plasma levels of sCD146 were less variable and better associated with the radiological severity of pulmonary congestion than BNP: Circulating sCD146 levels presented a stepwise increase with increasing degree of pulmonary congestion. Median plasma levels of sCD146 in patients without or with mild signs of congestion, interstitial edema, and alveolar edema were 316 ng/mL (IQR 249-388 ng/mL), 348 ng/mL (IQR 267-478 ng/mL), and 438 ng/mL (IQR 346-690 ng/mL), respectively. Based on ROC curve analysis, circulating sCD146 levels could discriminate between interstitial and alveolar edema better than BNP levels (AUC 0.71, 95% confidence interval 0.56-0.85 vs. 0.51, 95% confidence interval 0.34-0.68).
Fig. 2 shows the relationship between BNP levels, sCD146 levels, and myocardial necrosis. BNP levels were associated with the level of troponin T. When the population was split into three groups according to troponin T levels, BNP values were higher in the second and third tertiles of troponin T than the first tertile (
P<0.001). In contrast to BNP levels, sCD146 levels were not increased, and even slightly decreased, in the second and third tertiles of troponin T compared with baseline.
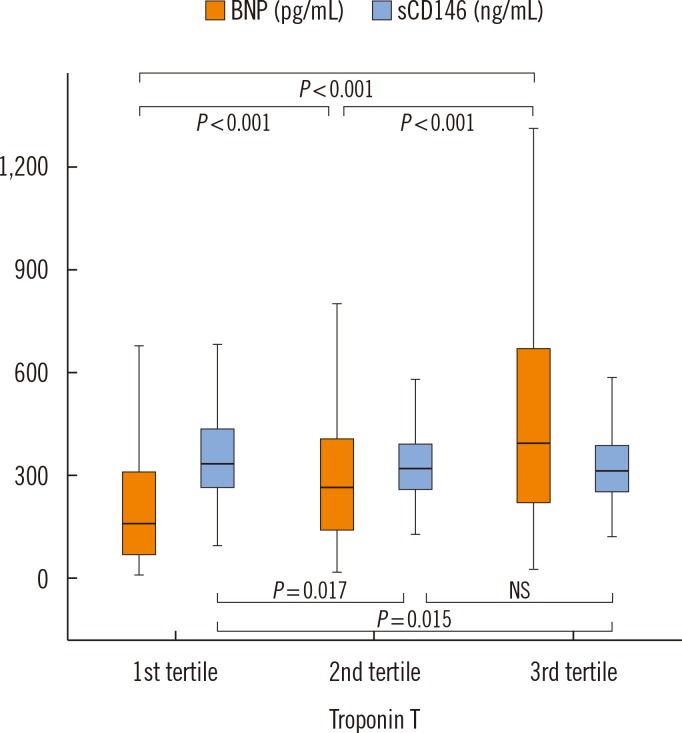 | Fig. 2
Plasma levels of BNP and sCD146 according to troponin T levels. Increasing levels of troponin T are associated with higher BNP, but similar or lower levels of sCD146. Median and interquartile range are displayed.
Abbreviations: BNP, brain natriuretic peptide; sCD146, soluble CD146; NS, not significant.

|
Go to :

DISCUSSION
The present study confirmed the potential of sCD146 as a biomarker for the assessment of systemic congestion. Indeed, in ACS patients, circulating sCD146 levels reflected the severity of pulmonary congestion without being affected by the level of myocardial necrosis.
CD146 is a component of the endothelial junction expressed in endothelial cells and its soluble form (sCD146) is considered as a marker of endothelial damage [
7]. We recently demonstrated that sCD146 positively correlated with the inferior vena cava diameter in patients with AHF [
5]. We further demonstrated that pulmonary edema was also positively correlated with the level of myocardial expression of CD146 in a pre-clinical model of sub-AHF [
5]. In this study, we showed a positive relationship between the level of sCD146 and the severity of pulmonary congestion in 927 patients with ACS. This might be related to a greater level of "chronic" endothelial dysfunction as suggested by the higher prevalence of peripheral vascular diseases and diabetes. The positive relationship between sCD146 and pulmonary congestion, observed a few hours after ACS onset, may also be related to the acute alteration of the ischemic left ventricle, leading to acute diastolic dysfunction, increase in post-capillary pulmonary pressure, and pulmonary edema. Further studies should assess the time course of sCD146, especially its decay after diuretic therapy, in relation to pulmonary congestion to ascertain the role of pulmonary vascular stretch in sCD146 release.
Our study also showed a positive relationship between troponin T and BNP levels, but not with sCD146. BNP is known to increase in the presence of myocardial ischemia [
34].
In contrast, the lack or even the negative association of sCD146 with myocardial necrosis is a novel observation. It suggests that cardiac myocytes are unlikely to be the main source of sCD146 in patients with ACS. Altogether, our data suggest an extra-cardiac source of sCD146 (possibly in the post-capillary pulmonary vessels) in patients with pulmonary edema.
Since relevant pulmonary congestion complicates the course of one of ten patients presenting with ACS and, as confirmed in our study, negatively influences short-term outcomes, sCD146 may help emergency physicians, cardiologists, and intensivists to assess and monitor pulmonary congestion in patients with ongoing ACS.
Our study has some limitations. First, data were derived from a single-center prospective cohort and the subgroups of patients presenting radiological evidence of pulmonary congestion, in particular alveolar edema, were rather small, despite a considerable number of included patients. A generalization of the findings may need confirmation in multi-centric cohorts. Secondly, pulmonary congestion was assessed by conventional chest radiography at admission by one experienced, certified radiologist. Although this is a common clinical practice, this method might not be very accurate in assessing pulmonary congestion. In particular, "hemodynamic congestion", which often precedes the redistribution of fluids into the lungs, may be present without radiological signs of pulmonary edema [
8]. The results of this study should be confirmed using alternative non-invasive (flow of the pulmonary vein, lung ultrasound, and CT scan) or invasive (pulmonary catheter) methods for the assessment of left-sided congestion [
9]. Third, this cohort did not specifically include patients combining ACS with other pulmonary diseases (e.g., pulmonary infection) and, therefore, no analysis of the performance of sCD146 for the differentiation of pulmonary opacities of different origins could be performed. However, since other causes of pulmonary opacity are much less frequent than pulmonary edema in patients with ACS, this differentiation could be of limited clinical value. Fourth, plasma sCD146 levels were measured in blood samples drawn immediately on hospital admission before PCI, whereas concentrations of troponin T and BNP were measured in blood samples collected exactly 24 hr after the onset of chest pain. This design was chosen to assess peak levels of both troponin T and BNP according to their dynamic of release.
In conclusion, the novel endothelial biomarker, sCD146, reflects the radiological severity of pulmonary congestion better than BNP in patients with ACS and, in contrast to BNP, is not affected by the amount of myocardial cell necrosis. Additional studies are needed in patients with AHF secondary to ACS and to other causes to confirm these results.
Go to :

Acknowledgments
This study was supported by the Project of Conceptual Development of Research Organization (Department of Health) 65269705 and by the European Regional Development Fund-Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123). MA is recipient of a fellowship of the Collège de Médecine des Hôpitaux de Paris.
Go to :

Notes
Go to :

References
1. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, OPTIMIZE-HF Investigators and Hospitals, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008; 168:847–854. PMID:
18443260.
2. Bahit MC, Lopes RD, Clare RM, Newby LK, Pieper KS, Van de Werf F, et al. Heart failure complicating non-ST-segment elevation acute coronary syndrome: timing, predictors, and clinical outcomes. JACC Heart Fail. 2013; 1:223–229. PMID:
24621874.
3. Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HD. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Investigators. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004; 110:3206–3212. PMID:
15533869.

4. Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004; 44:1988–1995. PMID:
15542281.

5. Gayat E, Caillard A, Laribi S, Mueller C, Sadoune M, Seronde MF, et al. Soluble CD146, a new endothelial biomarker of acutely decompensated heart failure. Int J Cardiol. 2015; 199:241–247. PMID:
26209827.

6. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012; 33:2551–2567. PMID:
22922414.

7. Bardin N, Moal V, Anfosso F, Daniel L, Brunet P, Sampol J, et al. Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb Haemost. 2003; 90:915–920. PMID:
14597988.

8. Picano E, Gargani L, Gheorghiade M. Why, when, and how to assess pulmonary congestion in heart failure: pathophysiological, clinical, and methodological implications. Heart Fail Rev. 2010; 15:63–72. PMID:
19504345.

9. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015; 17:544–558. PMID:
25999021.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download