This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Anti-H antibody is a type of anti-red blood cell (RBC) antibody that agglutinates with H antigen, which is universally present on human RBCs. The most widely known problem related to the anti-H antibody is the potentially lethal hemolytic transfusion reaction seen in individuals with the Bombay O blood type [
123]. Other anti-H antibodies are mostly found in A1 or A1B blood type individuals and are usually cold reactive and clinically benign [
3]. We report a Korean patient with an anti-H antibody exhibiting a wide thermal range.
A 79-yr-old woman diagnosed with pneumonia was due to receive a transfusion of RBCs in the Guro hospital of Korea University, Seoul, Korea. Her blood type was A, RhD+. However, the antibody-screening test was positive; an antibody identification test revealed 11 positive panels with no reaction to autologous RBCs by using the Ortho BioVue Innova system (Ortho-Clinical Diagnostics, Raritan, NJ, USA). The manufacturer's test RBCs used in the antibody screening and identification tests were O cells. ABO serotyping of the patient's serum with O cells showed strong agglutination. We suspected anti-IH or anti-H antibodies with wide thermal amplitude and conducted further evaluation [
4].
An ABO genotyping was performed for accurate genotype identification. Various types of RBCs from random donors were used, including autologous A
1, adult O, RhD- O, A
1, and enzyme-treated O cells. A
1 cells were tested with the patient's serum using both the column agglutination test (CAT) and tube method [
56]. Cord blood A
1, B, O, and A
1B cells were tested to rule out the possibility of anti-IH antibodies [
7]. Additionally, dithiothreitol (DTT)-treated serum was tested with O cells to specify the antibody's immunoglobulin type. The Ortho BioVue Innova system was used for the CAT; tests were carried out at room temperature and Coombs' phase where appropriate. All tests were conducted according to the procedures indicated in the AABB Technical Manual and with methods described by the relevant manufacturers [
3].
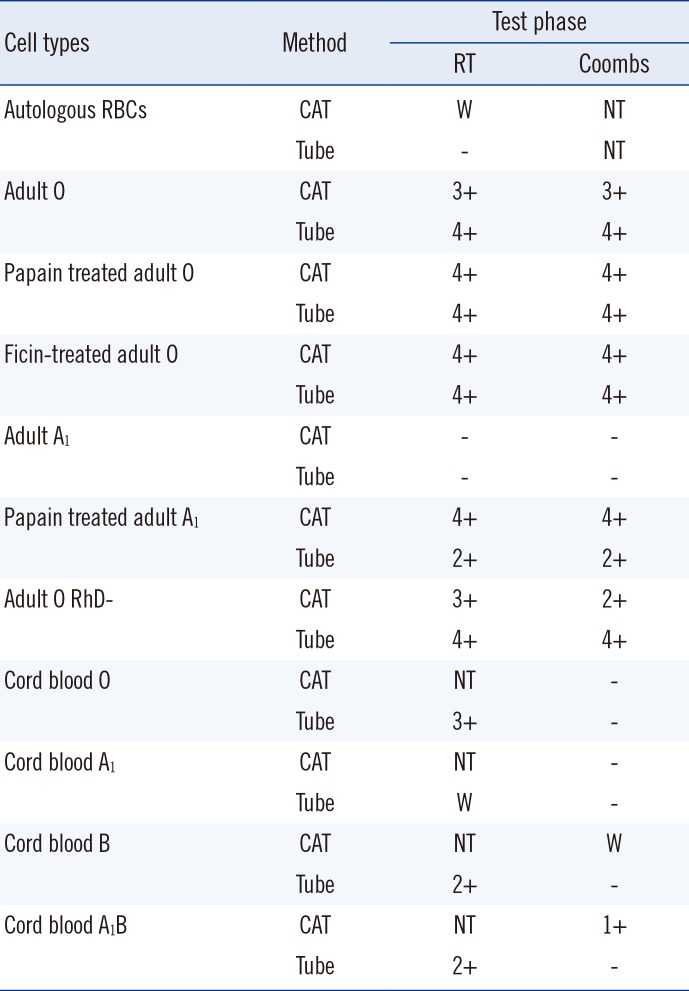
The patient was identified as having an A102/A102 genotype through sequence analysis. The antibody identification test showed 4+ in all panels through saline, 30 min cold incubation, albumin, and Coombs' phase; no agglutination with autologous RBCs was observed as noted above. The CAT of the patient's serum with adult A
1 cells showed no agglutination, including autologous RBCs. Tests with adult O cells revealed agglutination of 3+ or more in all phases. These results suggested the presence of anti-IH or anti-H antibodies, as did the strong reaction with H antigen-abundant O cells and weak or absent reactions with A
1 cells that lacked H antigens. Enzyme treatment of RBCs did not cause any significant changes in reactivity to O cells, while papain-treated A
1 cells showed agglutination of 2+ or more. The specific effect of enzyme treatment on A
1 cells in reaction with anti-H or anti-IH antibodies was unclear; results of this test did not favor any specific type of antibody (
Table 1).
Table 1
Column agglutination test with various RBCs
|
Cell types |
Method |
Test phase |
|
RT |
Coombs |
|
Autologous RBCs |
CAT |
W |
NT |
|
Tube |
- |
NT |
|
Adult O |
CAT |
3+ |
3+ |
|
Tube |
4+ |
4+ |
|
Papain treated adult O |
CAT |
4+ |
4+ |
|
Tube |
4+ |
4+ |
|
Ficin-treated adult O |
CAT |
4+ |
4+ |
|
Tube |
4+ |
4+ |
|
Adult A1
|
CAT |
- |
- |
|
Tube |
- |
- |
|
Papain treated adult A1
|
CAT |
4+ |
4+ |
|
Tube |
2+ |
2+ |
|
Adult O RhD- |
CAT |
3+ |
2+ |
|
Tube |
4+ |
4+ |
|
Cord blood O |
CAT |
NT |
- |
|
Tube |
3+ |
- |
|
Cord blood A1
|
CAT |
NT |
- |
|
Tube |
W |
- |
|
Cord blood B |
CAT |
NT |
W |
|
Tube |
2+ |
- |
|
Cord blood A1B |
CAT |
NT |
1+ |
|
Tube |
2+ |
- |

Cord blood A
1 cells showed weak reactions only in the tube method performed at room temperature. Neonatal RBCs have incomplete development of ABO antigens and have fewer H antigens on their surface compared with adult RBCs [
3]. Thus, these results suggested that the antibody reacted with a small amount of H antigen left on RBCs with an incomplete A
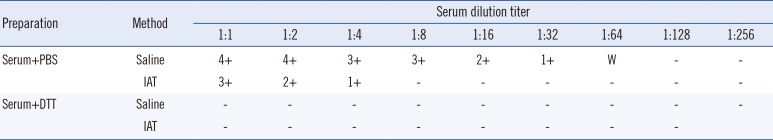
1 phenotype. Cord blood O cells showed 3+ reactions only at room temperature. The lack of agglutination in Coombs' phase was interpreted as a weakened reaction due to fewer H antigens on RBC surfaces. DTT-treated serum showed no agglutination with adult O cells in contrast to phosphate-buffered saline (PBS)-mixed control samples, as the treatment inactivated IgM, which were identified as cold antibodies with a sufficiently high titer to react in the Coombs' phase (
Table 2).
Table 2
Adult O cell with dithiothreitol-treated serum
|
Preparation |
Method |
Serum dilution titer |
|
1:1 |
1:2 |
1:4 |
1:8 |
1:16 |
1:32 |
1:64 |
1:128 |
1:256 |
|
Serum+PBS |
Saline |
4+ |
4+ |
3+ |
3+ |
2+ |
1+ |
W |
- |
- |
|
IAT |
3+ |
2+ |
1+ |
- |
- |
- |
- |
- |
- |
|
Serum+DTT |
Saline |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
IAT |
- |
- |
- |
- |
- |
- |
- |
- |
|

Anti-IH antibody reacts weakly or not at all with I-RBCs, and reacts strongly only in the presence of both I and H antigens [
78]. Cord blood RBCs lack I antigens; therefore, 3+ agglutination of cord blood O cells at room temperature signified the presence of anti-H antibodies. Adult Oi cells could have further confirmed this diagnosis; however, blood of that type was unavailable. The patient received a transfusion shortly after the identification test, and further evaluations at 4℃ could not be performed owing to insufficient sample amount.
In conclusion, we identified a case of anti-H antibody with a high titer, which was not reported in previous studies of the frequencies of unexpected antibodies in the Korean population [
910]. This report contributes to identification of anti-H antibodies in cases of unusual ABO discrepancies.

