This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Glucose meters are increasingly used in hospitals and home settings. These handheld devices provide instant readings and are therefore, beneficial for glucose-level monitoring in patients with diabetes [
1]. Nonetheless, there have been concerns regarding the measurement accuracy of glucose meters. Some substances, such as vitamin C and maltose, are considered responsible for errors in glucose measurements using point-of-care testing glucose meters [
123]. Vitamin C is widely used in the treatment of cancer, viral infections, severe burns, and chronic fatigue syndrome because of the antioxidant effects [
4]. Maltose can be upregulated in patients undergoing peritoneal dialysis involving icodextrin as an osmotic agent [
3]. High concentrations of vitamin C and maltose in blood can lead to false increase in glucose meter readings, resulting in misdiagnosis of hypoglycemia and potential fatalities [
1]. Therefore, there is an urgent need for interference-resistant glucose meters [
5]. We evaluated the interference of vitamin C and maltose with glucose readings obtained using the following three meters: Accuchek Inform (Roche Diagnostics, Indianapolis, IN, USA), Starstrip (Nova Biomedical, Waltham, MA, USA), and Barozen H plus (i-SENS Inc., Seoul, Korea). These three models are based on the glucose dehydrogenase-pyrroloquinoline quinone (GDH-PQQ), modified glucose oxidase (GOD), and GDH-flavin adenine dinucleotide (FAD) assays, respectively [
56].
After prior depletion of glucose by overnight storage at room temperature, we prepared three pooled blood specimens [
4] from EDTA-treated whole blood samples and added glucose (CAS 50-99-7, Duksan Pure Chemicals Co., Ansan, Korea) at final concentrations of 60, 126, or 300 mg/dL. To study the effect of various concentrations of vitamin C (0, 3, 15, or 30 mg/dL) [
7] or maltose (0, 10, 40, 200, or 500 mg/dL) [
8], we added 0.15 mL of the vitamin C or maltose stock solutions (control: normal saline) to 3 mL of pooled blood specimens. The stock solutions were prepared from L-ascorbic acid (CAS 50-81-7, Sigma-Aldrich, St. Louis, MO, USA) and D (+) -maltose monohydrate (CAS 6363-53-7, Junsei Chemical Co., Tokyo, Japan). Then, we compared the whole blood glucose readings of the specimens, with and without an interfering substance, using three glucose meters. We also measured the plasma glucose levels in each sample with Hitachi 7600 chemistry analyzer (Hitachi, Tokyo, Japan) for a benchmark comparison. Each sample was tested in duplicate. Assessment of interference and accuracy for each glucose meter was performed according to the criteria of the International Organization for Standardization (ISO) 15197:2013(E) [
9].
Accuchek Inform glucose level readings at all concentrations were subject to significant positive interference (>10% deviation from control sample levels) from vitamin C presence at concentrations of 15 and 30 mg/dL, whereas no significant positive interference was observed for Barozen H plus and Starstrip models (
Fig. 1). However, Starstrip failed to produce readings in two out of three measurements in specimens, which contained 30 mg/dL vitamin C (
Fig. 1). In contrast to the effect of vitamin C, no significant interference at any maltose concentration was noted for all the three models tested (
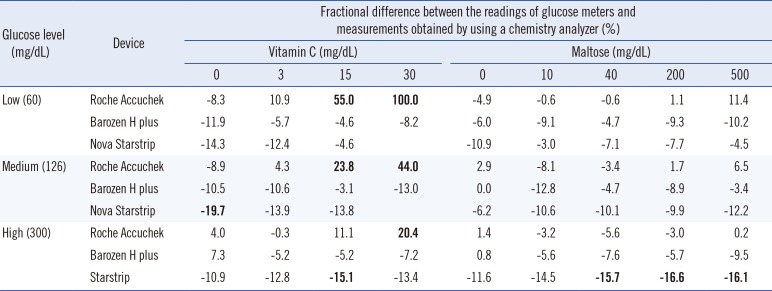
Fig. 1). Owing to interference from high concentrations of vitamin C (15 and 30 mg/dL), Accuchek Inform showed unacceptable accuracy levels (>15% difference from the value obtained by the chemistry analyzer;
Table 1). Starstrip generally showed lower readings than those determined by the chemistry analyzer, especially at higher glucose concentrations: a negative bias >15% was detected in four out of nine measurements (
Table 1).
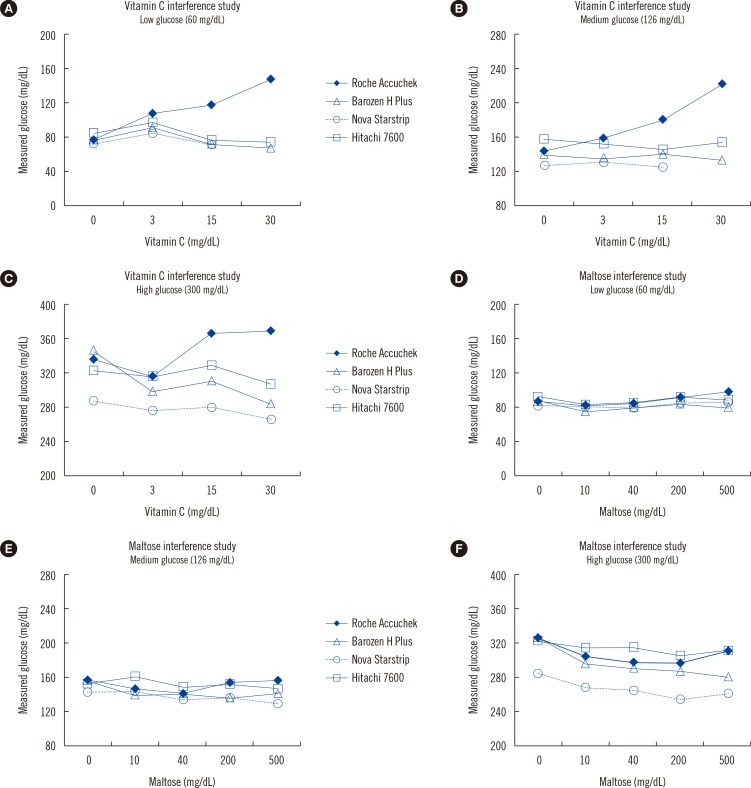 | Fig. 1Glucose level measurements in different concentrations of interferents. Glucose was added to blood at various concentrations: low (60 mg/dL), medium (126 mg/dL), and high (300 mg/dL). The X-axis represents the level of interferent, and the Y-axis represents the level of glucose. Experiments with vitamin C as an interferent are shown in (A), (B), and (C). Experiments with maltose are shown in (D), (E), and (F). Three models of glucose meters were evaluated: Accuchek Inform (Roche Diagnostics), Starstrip (Nova Biomedical), and Barozen H plus (i-SENS Inc.). Hitachi 7600 chemistry analyzer (Hitachi) was used for a benchmark comparison.
|
Table 1
Accuracy of whole-blood glucose concentration readings using three glucose meter models compared with the measurements obtained by using a chemistry analyzer
|
Glucose level (mg/dL) |
Device |
Fractional difference between the readings of glucose meters and measurements obtained by using a chemistry analyzer (%) |
|
Vitamin C (mg/dL) |
Maltose (mg/dL) |
|
0 |
3 |
15 |
30 |
0 |
10 |
40 |
200 |
500 |
|
Low (60) |
Roche Accuchek |
-8.3 |
10.9 |
55.0
|
100.0
|
-4.9 |
-0.6 |
-0.6 |
1.1 |
11.4 |
|
Barozen H plus |
-11.9 |
-5.7 |
-4.6 |
-8.2 |
-6.0 |
-9.1 |
-4.7 |
-9.3 |
-10.2 |
|
Nova Starstrip |
-14.3 |
-12.4 |
-4.6 |
|
-10.9 |
-3.0 |
-7.1 |
-7.7 |
-4.5 |
|
Medium (126) |
Roche Accuchek |
-8.9 |
4.3 |
23.8
|
44.0
|
2.9 |
-8.1 |
-3.4 |
1.7 |
6.5 |
|
Barozen H plus |
-10.5 |
-10.6 |
-3.1 |
-13.0 |
0.0 |
-12.8 |
-4.7 |
-8.9 |
-3.4 |
|
Nova Starstrip |
-19.7
|
-13.9 |
-13.8 |
|
-6.2 |
-10.6 |
-10.1 |
-9.9 |
-12.2 |
|
High (300) |
Roche Accuchek |
4.0 |
-0.3 |
11.1 |
20.4
|
1.4 |
-3.2 |
-5.6 |
-3.0 |
0.2 |
|
Barozen H plus |
7.3 |
-5.2 |
-5.2 |
-7.2 |
0.8 |
-5.6 |
-7.6 |
-5.7 |
-9.5 |
|
Starstrip |
-10.9 |
-12.8 |
-15.1
|
-13.4 |
-11.6 |
-14.5 |
-15.7
|
-16.6
|
-16.1
|

Vitamin C is a strong antioxidant that inactivates free radicals and can be oxidized at the surface of electrochemical strips producing electrons and increasing the current [
1]. Icodextrin, an osmotic agent used in peritoneal dialysis, is metabolized in the systemic circulation into various glucose polymers, mainly maltose [
10]. It can interfere with readings obtained using GDH-PQQ-based method, because GDH-PQQ catalyzes the oxidation of not only glucose but also other sugars [
1]. Thus, vitamin C and maltose can cause positive interference resulting in misdiagnosis of true glucose levels. However, GDH currently used in Accuchek Inform was modified by the manufacturer to increase enzyme specificity for glucose and to diminish probability of incorrect high glucose readings [
8]. In this study, all three glucose meters showed reliable results in presence of maltose. However, at higher vitamin C concentrations, Accuchek Inform showed a positive bias, while Starstrip occasionally malfunctioned.
In conclusion, high concentrations of vitamin C may affect blood glucose measurements depending on the glucose meter used. Therefore, caution is required while monitoring the glucose level, using a glucose meter, of patients receiving high dose of vitamin C. There is a need for continuous technical improvement and further studies for possible interferents of glucose meters.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download