Dear Editor,
Philadelphia chromosome-positive (Ph+) or BCR-ABL1-positive AML accounts for <1% of newly diagnosed AML cases [1]. Although the role of Ph in CML pathogenesis has been elucidated, Ph+ AML pathogenesis remains controversial. Ph+ AML is believed to represent the de novo myeloid blast phase (BP) of CML and, therefore, was not recognized in the 2008 WHO classification of myeloid neoplasms and acute leukemia [2].
Konoplev et al. [3] presented molecular evidence suggesting that Ph+ AML is a clinicopathological entity distinct from CML-BP, in a study involving nine patients with Ph+ AML and five patients with CML-BP at their initial presentations. They showed that patients with Ph+ AML carried the nucleophosmin (NPM1) mutation at a similar frequency as that seen in all cases of AML, and that they lacked ABL1 mutations that occur in a proportion of patients with CML-BP. Internal tandem duplication (ITD) of the Fms-like receptor tyrosine kinase 3 (FLT3) was detected in a Ph+ AML patient, but no Ph+ AML patients harbored both the NPM1 mutation and the FLT3 ITD. We present a rare case of BCR-ABL1-positive AML with both the NPM1 mutation and the FLT3 ITD.
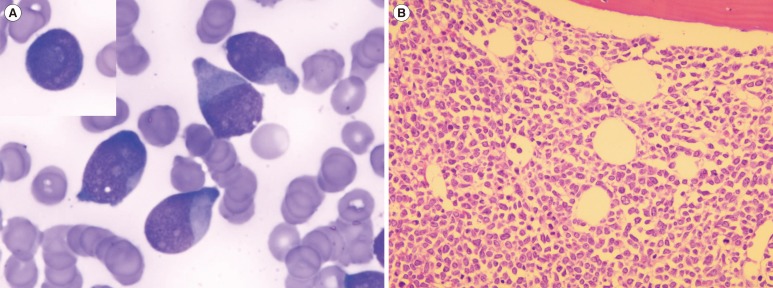
A 79-yr-old woman was referred to our hospital after several months of general weakness and weight loss. She had undergone surgery for acute appendicitis one year earlier. Her initial white blood cell count was 157.5×109/L (80% blasts and 1% basophils), hemoglobin was 11.8 g/dL, and platelet count was 87×109/L. She did not show organomegaly or lymphadenopathy. Her bone marrow (BM) was packed with blasts (92.3% of all nucleated cells) with cup-like nuclear indentation (Fig. 1) and showed no significant dyshematopoietic features. Flow cytometry revealed that the blasts were positive for CD13 (94%), CD33 (98%), CD34 (72%), HLA-DR (46%), and cytoplasmic myeloperoxidase (86%). Chromosome analysis revealed t(2;5)(p12;p15) in 20 metaphases, which was considered a constitutional chromosomal abnormality. Multiplex reverse transcription (RT)-PCR using HemaVision kit (DNA Diagnostic A/S, Risskov, Denmark) revealed the presence of minor BCR-ABL1 (e1a2) fusion transcripts. Interphase FISH analysis of formalin-fixed paraffin-embedded tissue from BM trephine biopsy section showed no fusion signals by using locus specific identifier BCR/ABLdual-color, dual-fusion translocation probe set (Abbott Molecular/Vysis, Des Plaines, IL, USA).
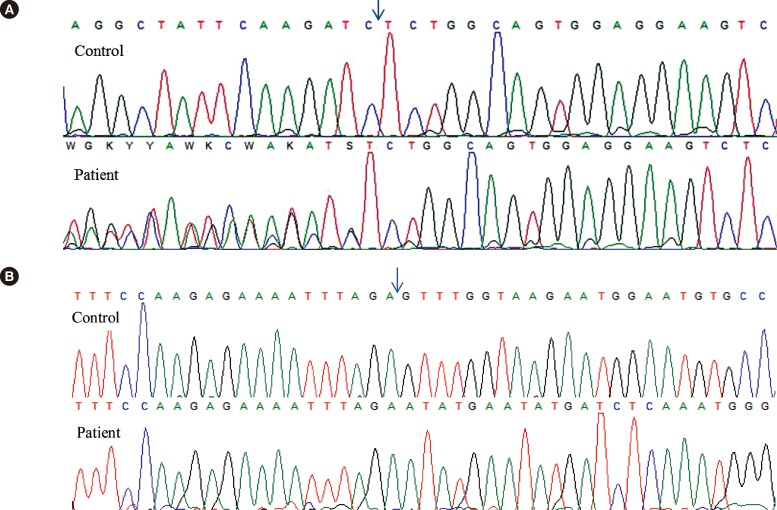
Genomic DNA was amplified to detect the FLT3 ITD and NPM1 exon 12 mutations. Total RNA was extracted and RT-PCR was carried out to amplify the BCR/ABL kinase domain. Direct sequencing of the amplified products revealed that our patient had type A mutations of NPM1 (Fig. 2A) and FLT3 ITD (Fig. 2B), but no ABL1 mutations.
The patient was diagnosed as having AML without maturation, according to the 2008 WHO classification of haematopoietic and lymphoid tumors [4], and was considered to have a cryptic e1a2 BCR-ABL1 fusion. Although cytarabine and decitabine therapy was started, the patient died one week after AML diagnosis.
The clinicopathological features that suggested Ph+ AML rather than CML-BP included the absence of a clinical history of hematologic disorders (including CML and MDS); no dwarf megakaryocytes, splenomegaly, or basophilia; and the absence of an extra Ph, trisomy 8, trisomy 19, or isochromosome 17q [35]. This case showed clinical and laboratory features compatible with Ph+ AML, as mentioned above.
Considering the findings of Konoplev's cases [3] and this case, NPM1 mutations and FLT3 ITD may be concurrent genetic abnormalities in patients with Ph+ AML. The coexistence of the FLT3 ITD and the NPM1 mutations, as observed in our patient, is reported in about 40% of patients with AML and mutated NPM1 [6]. This case supports the idea that Ph+ AML is a distinct entity. Another study also showed that NPM1 mutations occur only in patients with AML and not in those with CML, whereas ABL1 mutations are common in patients with CML [7]. Furthermore, a high-resolution array-based comparative genomic hybridization study indicated that a unique loss within immunoglobulin genes provides a simple test to differentiate clinically similar de novo Ph+ AML cases from CML-BP cases [1].
To our knowledge, our patient is the first case of Ph+ AML with both FLT3 ITD and NPM1 mutations. Ph+ AML cases seem to have different molecular or genomic features from those with CML-BP [138]. However, to describe Ph+ AML as a discrete entity in the WHO classification, it is important to document more cases and perform more aggressive diagnostic workups, including FLT3 ITD and NPM1 mutational analyses and analysis of immunoglobulin genes. Especially, a hematopathologist should keep in mind that cup-like nuclei and fish mouth nuclei may represent an important morphologic clue to provoke testing for both the FLT3 ITD and the NPM1 mutations [910].
References
1. Nacheva EP, Grace CD, Brazma D, Gancheva K, Howard-Reeves J, Rai L, et al. Does BCR/ABL1 positive acute myeloid leukaemia exist? Br J Haematol. 2013; 161:541–550. PMID: 23521501.
2. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114:937–951. PMID: 19357394.

3. Konoplev S, Yin CC, Kornblau SM, Kantarjian HM, Konopleva M, Andreeff M, et al. Molecular characterization of de novo Philadelphia chromosome-positive acute myeloid leukemia. Leuk Lymphoma. 2013; 54:138–144. PMID: 22691121.
4. Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008. p. 130–139.
5. Soupir CP, Vergilio JA, Dal Cin P, Muzikansky A, Kantarjian H, Jones D, et al. Philadelphia chromosome-positive acute myeloid leukemia: a rare aggressive leukemia with clinicopathologic features distinct from chronic myeloid leukemia in myeloid blast crisis. Am J Clin Pathol. 2007; 127:642–650. PMID: 17369142.
6. Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005; 352:254–266. PMID: 15659725.

7. Watkins DB, Hughes TP, White DL, D'Andrea RJ. NPM1 mutations occur rarely or not at all in chronic myeloid leukaemia patients in chronic phase or blast crisis. Leukemia. 2013; 27:489–490. PMID: 22791379.
8. Bacher U, Haferlach T, Alpermann T, Zenger M, Hochhaus A, Beelen DW, et al. Subclones with the t(9;22)/BCR-ABL1 rearrangement occur in AML and seem to cooperate with distinct genetic alterations. Br J Haematol. 2011; 152:713–720. PMID: 21275954.
9. Park BG, Chi HS, Jang S, Park CJ, Kim DY, Lee JH, et al. Association of cup-like nuclei in blasts with FLT3 and NPM1 mutations in acute myeloid leukemia. Ann Hematol. 2013; 92:451–457. PMID: 23238897.
10. Jain P, Vega-Vazquez F, Faderl S. "Cup-like" blasts and NPM1 and FLT3 (ITD) mutations in acute myeloid leukemia (AML). Int J Hematol. 2013; 98:3. PMID: 23690292.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download