This article has been
cited by other articles in ScienceCentral.
Abstract
We established age- and gender-specific reference ranges for the 36 routine complete blood cell (CBC) and 57 cell population data (CPD) items in the Sysmex XN-2000 (Sysmex, Japan). In total, 280 peripheral blood samples were obtained from an equal number of healthy adults. Values for 36 routine items and 57 CPD items were obtained for each sample, and the results were categorized into six subgroups (N>39 in each subgroup) according to patient age (20-40, 41-60, and >60 yr) and gender (male and female), and compared with respect to age and gender differences. The majority of data items (22 of 36 routine CBC items and 44 of 57 CPD items) exhibited significant differences (P≤0.05) in their results with respect to age or gender, and several red cell-, lymphocyte-, and platelet-related data tended to decrease in women or older adults. These results provide a basis for establishing age- and gender-specific reference ranges for routine and CPD items in Sysmex XN-2000. Furthermore, these reference ranges could be used to determine clinical significance for new items of Sysmex XN-2000 in further studies.
Keywords: Age, Automated blood cell analyzer, Gender, Item, Reference range, Sysmex XN-2000
The recently launched automated blood cell analyzer, Sysmex XN-2000 (Sysmex, Kobe, Japan), can measure 36 routine and 57 various cell population data (CPD) items, providing a detailed image of the cells examined. Furthermore, this analyzer can be used to derive clinical information. Therefore, the establishment of age- and gender-specific reference ranges for both routine and CPD items is critical in the accurate assessment of a patient's clinical status. This investigation would be also useful to determine clinical significance of routine and CPD items as biomarkers in various diseases. A recent study reported ethical differences in the reference ranges of complete blood cell (CBC) counts applicable to the Sysmex XE-2100 (Sysmex), while considering age and gender [
1]. The age-related increase in red cell distribution width and mean corpuscular volume in the CELL-DYN Sapphire (Abbott Diagnostics Division, Santa Clara, CA, USA) was also reported [
2]. In addition, the complex biologic profile of hematologic markers obtained from HmX (Beckman Coulter., Miami, FL, USA) or CA-500 (Sysmex) analyzers across different age subgroups were evaluated in the recent study [
3].
Although the reference ranges applicable to research-related items in the XE-2100 and reference range for immature platelet fraction (IPF) in the new XN-2000 analyzer with large study population were analyzed in the previous studies [
45], the establishment of age- and gender-specific reference ranges for all 36 routine and 57 CPD items in the new XN-2000 analyzer has not been performed. Therefore, we established age- and gender-specific reference ranges for the routine CBC and CPD items in the new Sysmex XN-2000.
In total, 280 peripheral blood samples were obtained from an equal number of healthy adults who received a general health examination at the Asan Medical Center, Seoul, Korea from April 2013 to June 2013 and were enrolled in this study. Inclusion criteria were as follows: (1) non-smoking individuals who showed normal CBC, (2) routine chemistry and urine analysis results without drug history, and (3) absence of other abnormal findings in their medical history or during physical examination. Samples were obtained with an EDTA vacutainer and were analyzed by using the Sysmex XN-2000 with less than 2 hr between venipuncture and testing. Values for 36 routine and 57 CPD items were obtained for each sample. The results were categorized into six subgroups (number of data in each subgroup: 43, 50 and 51 in age 20-40, 41-60, and >60, respectively [for males]; 52, 44, and 40 in age 20-40, 41-60, and >60, respectively [for females]) according to patient age [(1) 20-40, (2) 41-60, and (3) >60 yr] and gender (male and female), and were compared with respect to age and gender by the Mann-Whitney U test (for two subgroups) or the Kruskal-Wallis test (for three subgroups). In these comparisons,
P values ≤0.05 were considered statistically significant. The stratified reference ranges in each item were calculated according to the CLSI guidelines for determination of reference ranges in clinical laboratory tests [
6]. Because all subgroups of each item did not show a normal distribution by Kolmogorov-Smirnov test, the 2.5th-97.5th percentile of each item was determined as a reference range. Over the analytical phase, quality control procedures were performed by using internal quality control (IQC) materials provided by the manufacturer twice a day for the 36 routine items.
Of the 36 routine items, 22 (61.1%) items showed significant differences in their values with respect to age (10 items), gender (5 items), or both (7 items). These items included all CBC items except for white blood cell counts; platelet distribution width, mean platelet volume, thrombocrit, absolute number of lymphocytes, monocytes, eosinophils, proportion of monocytes and eosinophils, all items related to reticulocytes, and reticulated hemoglobin. The remaining 14 items did not exhibit significant differences in their values with respect to either age or gender.
Of the 57 CPD items, 44 (77.2%) items showed significant differences in their values with respect to age (9 items), gender (14 items), or both (21 items). The remaining 13 items did not exhibit significant differences in their values with respect to age or gender. These included items related to basophil counts, proportion and absolute number of particles obtained by subtracting immature granulocyte counts from the neutrophil counts (delta-NEUT), proportion of particles obtained by subtracting the high fluorescence lymphocyte count from the lymphocyte counts (delta-LYMPH), lateral and forward scattered light intensity of neutrophil area (NE-SSC and NE-FSC), fluorescence light distribution width of the neutrophil area (NE-WY), lateral scattered light distribution width of the monocyte area (MO-WX), delta-hemoglobin, and the ratio to the total platelet count of the count of platelets that appear in the area of stronger fluorescent light intensity within the IPF area of the platelet scattergram (H-IPF). Comparison results of the 36 routine and 57 CPD items between the gender subgroups are summarized in the
Supplemental Tables 1 and
2, respectively. Comparison results of 36 routine and 57 CPD items among three different age subgroups are described in the
Supplemental Tables 3 and
4, respectively.
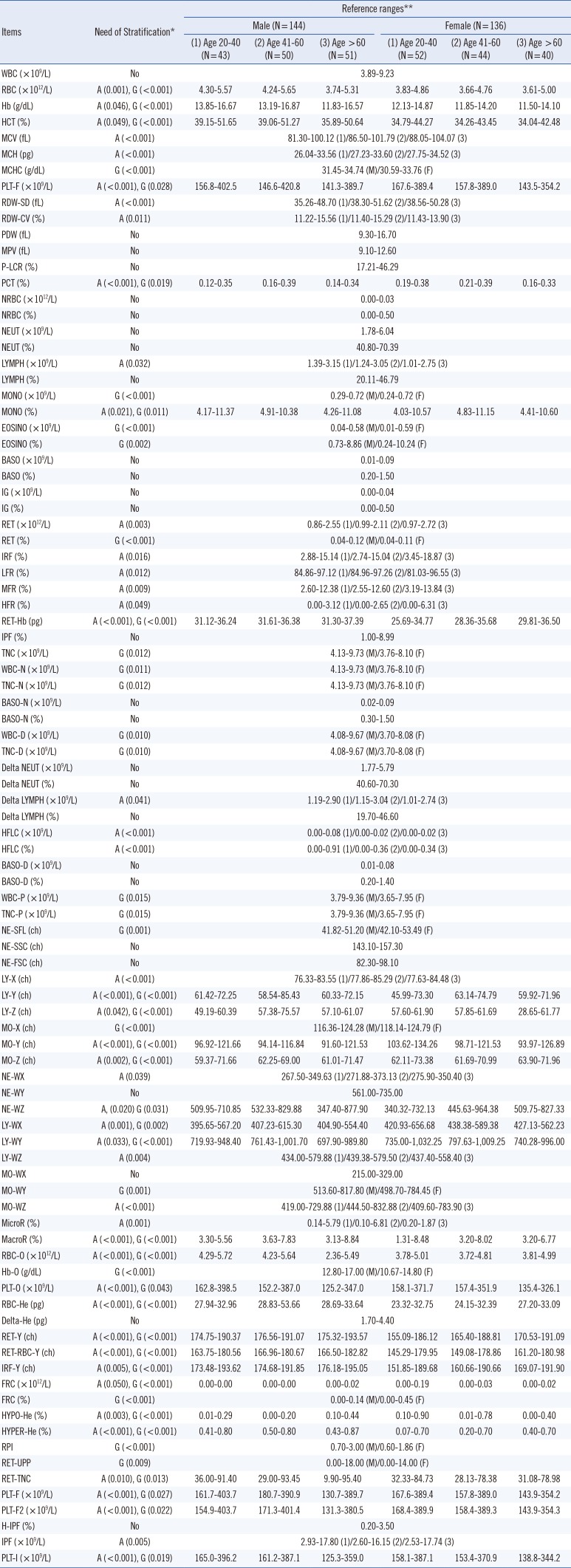
The stratified age- and gender-specific reference ranges were established, and these results are represented in
Table 1. We observed that several items related to red blood cell, lymphocytes, and platelet counts seem to be lower in women or old age subgroups than in men or young age subgroups. Our study also showed significant age-related increase in mean corpuscular volume/mean corpuscular hemoglobin, significant gender-related decrease (lower in females than males) in hemoglobin/hematocrit, significant age-related decrease in platelets (all
P<0.001) and constant white blood cells over life, which are consistent with previous studies [
23]. Although our present data satisfy the minimum sample number (N=39) to estimate 2.5 percentile or 97.5 percentile [
6], this number is not sufficient for the establishment of reference range itself, and our study data should be interpreted with caution from the definite shortage of numbers and inability to present 95% confidence intervals. Therefore, the generalizability of our reference range data needs to be validated in a comprehensive future study.
Table 1
Establishment of the reference ranges for all 93 items obtained from the XN-2000 automated blood cell analyzer
|
Items |
Need of Stratification*
|
Reference ranges**
|
|
Male (N=144) |
Female (N = 136) |
(1) Age 20-40
(N = 43) |
(2) Age 41-60
(N = 50) |
(3) Age > 60
(N = 51) |
(1) Age 20-40
(N = 52) |
(2) Age 41-60
(N = 44) |
(3) Age > 60
(N = 40) |
|
WBC ( × 109/L) |
No |
3.89-9.23 |
|
RBC ( × 1012/L) |
A (0.001), G ( < 0.001) |
4.30-5.57 |
4.24-5.65 |
3.74-5.31 |
3.83-4.86 |
3.66-4.76 |
3.61-5.00 |
|
Hb (g/dL) |
A (0.046), G ( < 0.001) |
13.85-16.67 |
13.19-16.87 |
11.83-16.57 |
12.13-14.87 |
11.85-14.20 |
11.50-14.10 |
|
HCT (%) |
A (0.049), G ( < 0.001) |
39.15-51.65 |
39.06-51.27 |
35.89-50.64 |
34.79-44.27 |
34.26-43.45 |
34.04-42.48 |
|
MCV (fL) |
A ( < 0.001) |
81.30-100.12 (1)/86.50-101.79 (2)/88.05-104.07 (3) |
|
MCH (pg) |
A ( < 0.001) |
26.04-33.56 (1)/27.23-33.60 (2)/27.75-34.52 (3) |
|
MCHC (g/dL) |
G ( < 0.001) |
31.45-34.74 (M)/30.59-33.76 (F) |
|
PLT-F ( × 109/L) |
A ( < 0.001), G (0.028) |
156.8-402.5 |
146.6-420.8 |
141.3-389.7 |
167.6-389.4 |
157.8-389.0 |
143.5-354.2 |
|
RDW-SD (fL) |
A ( < 0.001) |
35.26-48.70 (1)/38.30-51.62 (2)/38.56-50.28 (3) |
|
RDW-CV (%) |
A (0.011) |
11.22-15.56 (1)/11.40-15.29 (2)/11.43-13.90 (3) |
|
PDW (fL) |
No |
9.30-16.70 |
|
MPV (fL) |
No |
9.10-12.60 |
|
P-LCR (%) |
No |
17.21-46.29 |
|
PCT (%) |
A ( < 0.001), G (0.019) |
0.12-0.35 |
0.16-0.39 |
0.14-0.34 |
0.19-0.38 |
0.21-0.39 |
0.16-0.33 |
|
NRBC ( × 1012/L) |
No |
0.00-0.03 |
|
NRBC(%) |
No |
0.00-0.50 |
|
NEUT ( × 109/L) |
No |
1.78-6.04 |
|
NEUT (%) |
No |
40.80-70.39 |
|
LYMPH ( × 109/L) |
A (0.032) |
1.39-3.15 (1)/1.24-3.05 (2)/1.01-2.75 (3) |
|
LYMPH (%) |
No |
20.11-46.79 |
|
MONO ( × 109/L) |
G ( < 0.001) |
0.29-0.72 (M)/0.24-0.72 (F) |
|
MONO (%) |
A (0.021), G (0.011) |
4.17-11.37 |
4.91-10.38 |
4.26-11.08 |
4.03-10.57 |
4.83-11.15 |
4.41-10.60 |
|
EOSINO ( × 109/L) |
G ( < 0.001) |
0.04-0.58 (M)/0.01-0.59 (F) |
|
EOSINO (%) |
G (0.002) |
0.73-8.86 (M)/0.24-10.24 (F) |
|
BASO ( × 109/L) |
No |
0.01-0.09 |
|
BASO (%) |
No |
0.20-1.50 |
|
IG ( × 109/L) |
No |
0.00-0.04 |
|
IG (%) |
No |
0.00-0.50 |
|
RET ( × 1012/L) |
A (0.003) |
0.86-2.55 (1)/0.99-2.11 (2)/0.97-2.72 (3) |
|
RET (%) |
G ( < 0.001) |
0.04-0.12 (M)/0.04-0.11 (F) |
|
IRF (%) |
A (0.016) |
2.88-15.14 (1)/2.74-15.04 (2)/3.45-18.87 (3) |
|
LFR (%) |
A (0.012) |
84.86-97.12 (1)/84.96-97.26 (2)/81.03-96.55 (3) |
|
MFR (%) |
A (0.009) |
2.60-12.38 (1)/2.55-12.60 (2)/3.19-13.84 (3) |
|
HFR (%) |
A (0.049) |
0.00-3.12 (1)/0.00-2.65 (2)/0.00-6.31 (3) |
|
RET-Hb (pg) |
A ( < 0.001), G ( < 0.001) |
31.12-36.24 |
31.61-36.38 |
31.30-37.39 |
25.69-34.77 |
28.36-35.68 |
29.81-36.50 |
|
IPF (%) |
No |
1.00-8.99 |
|
TNC ( × 109/L) |
G (0.012) |
4.13-9.73 (M)/3.76-8.10 (F) |
|
WBC-N ( × 109/L) |
G (0.011) |
4.13-9.73 (M)/3.76-8.10 (F) |
|
TNC-N ( × 109/L) |
G (0.012) |
4.13-9.73 (M)/3.76-8.10 (F) |
|
BASO-N ( × 109/L) |
No |
0.02-0.09 |
|
BASO-N (%) |
No |
0.30-1.50 |
|
WBC-D ( × 109/L) |
G (0.010) |
4.08-9.67 (M)/3.70-8.08 (F) |
|
TNC-D ( × 109/L) |
G (0.010) |
4.08-9.67 (M)/3.70-8.08 (F) |
|
Delta NEUT ( × 109/L) |
No |
1.77-5.79 |
|
Delta NEUT (%) |
No |
40.60-70.30 |
|
Delta LYMPH ( × 109/L) |
A (0.041) |
1.19-2.90 (1)/1.15-3.04 (2)/1.01-2.74 (3) |
|
Delta LYMPH (%) |
No |
19.70-46.60 |
|
HFLC ( × 109/L) |
A ( < 0.001) |
0.00-0.08 (1)/0.00-0.02 (2)/0.00-0.02 (3) |
|
HFLC (%) |
A ( < 0.001) |
0.00-0.91 (1)/0.00-0.36 (2)/0.00-0.34 (3) |
|
BASO-D ( × 109/L) |
No |
0.01-0.08 |
|
BASO-D (%) |
No |
0.20-1.40 |
|
WBC-P ( × 109/L) |
G (0.015) |
3.79-9.36 (M)/3.65-7.95 (F) |
|
TNC-P ( × 109/L) |
G (0.015) |
3.79-9.36 (M)/3.65-7.95 (F) |
|
NE-SFL (ch) |
G (0.001) |
41.82-51.20 (M)/42.10-53.49 (F) |
|
NE-SSC (ch) |
No |
143.10-157.30 |
|
NE-FSC (ch) |
No |
82.30-98.10 |
|
LY-X (ch) |
A ( < 0.001) |
76.33-83.55 (1)/77.86-85.29 (2)/77.63-84.48 (3) |
|
LY-Y (ch) |
A ( < 0.001), G ( < 0.001) |
61.42-72.25 |
58.54-85.43 |
60.33-72.15 |
45.99-73.30 |
63.14-74.79 |
59.92-71.96 |
|
LY-Z (ch) |
A (0.042), G ( < 0.001) |
49.19-60.39 |
57.38-75.57 |
57.10-61.07 |
57.60-61.90 |
57.85-61.69 |
28.65-61.77 |
|
MO-X (ch) |
G ( < 0.001) |
116.36-124.28 (M)/118.14-124.79 (F) |
|
MO-Y (ch) |
A ( < 0.001), G ( < 0.001) |
96.92-121.66 |
94.14-116.84 |
91.60-121.53 |
103.62-134.26 |
98.71-121.53 |
93.97-126.89 |
|
MO-Z (ch) |
A (0.002), G ( < 0.001) |
59.37-71.66 |
62.25-69.00 |
61.01-71.47 |
62.11-73.38 |
61.69-70.99 |
63.90-71.96 |
|
NE-WX |
A (0.039) |
267.50-349.63 (1)/271.88-373.13 (2)/275.90-350.40 (3) |
|
NE-WY |
No |
561.00-735.00 |
|
NE-WZ |
A, (0.020) G (0.031) |
509.95-710.85 |
532.33-829.88 |
347.40-877.90 |
340.32-732.13 |
445.63-964.38 |
509.75-827.33 |
|
LY-WX |
A (0.001), G (0.002) |
395.65-567.20 |
407.23-615.30 |
404.90-554.40 |
420.93-656.68 |
438.38-589.38 |
427.13-562.23 |
|
LY-WY |
A (0.033), G ( < 0.001) |
719.93-948.40 |
761.43-1,001.70 |
697.90-989.80 |
735.00-1,032.25 |
797.63-1,009.25 |
740.28-996.00 |
|
LY-WZ |
A (0.004) |
434.00-579.88 (1)/439.38-579.50 (2)/437.40-558.40 (3) |
|
MO-WX |
No |
215.00-329.00 |
|
MO-WY |
G (0.001) |
513.60-817.80 (M)/498.70-784.45 (F) |
|
MO-WZ |
A ( < 0.001) |
419.00-729.88 (1)/444.50-832.88 (2)/409.60-783.90 (3) |
|
MicroR (%) |
A (0.001) |
0.14-5.79 (1)/0.10-6.81 (2)/0.20-1.87 (3) |
|
MacroR (%) |
A ( < 0.001), G ( < 0.001) |
3.30-5.56 |
3.63-7.83 |
3.13-8.84 |
1.31-8.48 |
3.20-8.02 |
3.20-6.77 |
|
RBC-O ( × 1012/L) |
A ( < 0.001), G ( < 0.001) |
4.29-5.72 |
4.23-5.64 |
2.36-5.49 |
3.78-5.01 |
3.72-4.81 |
3.81-4.99 |
|
Hb-O (g/dL) |
G ( < 0.001) |
12.80-17.00 (M)/10.67-14.80 (F) |
|
PLT-O ( × 109/L) |
A ( < 0.001), G (0.043) |
162.8-398.5 |
152.2-387.0 |
125.2-347.0 |
158.1-371.7 |
157.4-351.9 |
135.4-326.1 |
|
RBC-He (pg) |
A ( < 0.001), G ( < 0.001) |
27.94-32.96 |
28.83-53.66 |
28.69-33.64 |
23.32-32.75 |
24.15-32.39 |
27.20-33.09 |
|
Delta-He (pg) |
No |
1.70-4.40 |
|
RET-Y (ch) |
A ( < 0.001), G ( < 0.001) |
174.75-190.37 |
176.56-191.07 |
175.32-193.57 |
155.09-186.12 |
165.40-188.81 |
170.53-191.09 |
|
RET-RBC-Y (ch) |
A ( < 0.001), G ( < 0.001) |
163.75-180.56 |
166.96-180.67 |
166.50-182.82 |
145.29-179.95 |
149.08-178.86 |
161.20-180.98 |
|
IRF-Y (ch) |
A (0.005), G ( < 0.001) |
173.48-193.62 |
174.68-191.85 |
176.18-195.05 |
151.85-189.68 |
160.66-190.66 |
169.07-191.90 |
|
FRC ( × 1012/L) |
A (0.050), G ( < 0.001) |
0.00-0.00 |
0.00-0.00 |
0.00-0.02 |
0.00-0.19 |
0.00-0.03 |
0.00-0.02 |
|
FRC (%) |
G (<0.001) |
0.00-0.14 (M)/0.00-0.45 (F) |
|
HYPO-He (%) |
A (0.003), G ( < 0.001) |
0.01-0.29 |
0.00-0.20 |
0.10-0.44 |
0.10-0.90 |
0.01-0.78 |
0.00-0.40 |
|
HYPER-He (%) |
A ( < 0.001), G ( < 0.001) |
0.41-0.80 |
0.50-0.80 |
0.43-0.87 |
0.07-0.70 |
0.20-0.70 |
0.40-0.70 |
|
RPI |
G ( < 0.001) |
0.70-3.00 (M)/0.60-1.86 (F) |
|
RET-UPP |
G (0.009) |
0.00-18.00 (M)/0.00-14.00 (F) |
|
RET-TNC |
A (0.010), G (0.013) |
36.00-91.40 |
29.00-93.45 |
9.90-95.40 |
32.33-84.73 |
28.13-78.38 |
31.08-78.98 |
|
PLT-F ( × 109/L) |
A ( < 0.001), G (0.027) |
161.7-403.7 |
180.7-390.9 |
130.7-389.7 |
167.6-389.4 |
157.8-389.0 |
143.9-354.2 |
|
PLT-F2(×109/L) |
A ( < 0.001), G (0.022) |
154.9-403.7 |
171.3-401.4 |
131.3-380.5 |
168.4-389.9 |
158.4-389.3 |
143.9-354.3 |
|
H-IPF (%) |
No |
0.20-3.50 |
|
IPF ( × 109/L) |
A (0.005) |
2.93-17.80 (1)/2.60-16.15 (2)/2.53-17.74 (3) |
|
PLT-I ( × 109/L) |
A ( < 0.001), G (0.019) |
165.0-396.2 |
161.2-387.1 |
125.3-359.0 |
158.1-387.1 |
153.4-370.9 |
138.8-344.2 |

In conclusion, the majority of items (22 of 36 routine items and 43 of 57 CPD items) in the Sysmex XN-2000 exhibited significant differences in their results in healthy adults with respect to age or gender, and several red cell-, lymphocyte-, and platelet-related data tended to be decreased in women or older adults. Our study provides the basis for establishing age- and gender-specific reference ranges for routine and CPD items in Sysmex XN-2000, which could help determine the clinical significance for new items of the Sysmex XN-2000.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download