Abstract
Background
Automated Mediace Treponema pallidum latex agglutination (TPLA) and Mediace rapid plasma reagin (RPR) assays are used by many laboratories for syphilis diagnosis. This study compared the results of the traditional syphilis screening algorithm and a reverse algorithm using automated Mediace RPR or Mediace TPLA as first-line screening assays in subjects undergoing a health checkup.
Methods
Samples from 24,681 persons were included in this study. We routinely performed Mediace RPR and Mediace TPLA simultaneously. Results were analyzed according to both the traditional algorithm and reverse algorithm. Samples with discordant results on the reverse algorithm (e.g., positive Mediace TPLA, negative Mediace RPR) were tested with Treponema pallidum particle agglutination (TPPA).
Results
Among the 24,681 samples, 30 (0.1%) were found positive by traditional screening, and 190 (0.8%) by reverse screening. The identified syphilis rate and overall false-positive rate according to the traditional algorithm were lower than those according to the reverse algorithm (0.07% and 0.05% vs. 0.64% and 0.13%, respectively). A total of 173 discordant samples were tested with TPPA by using the reverse algorithm, of which 140 (80.9%) were TPPA positive.
Conclusions
Despite the increased false-positive results in populations with a low prevalence of syphilis, the reverse algorithm detected 140 samples with treponemal antibody that went undetected by the traditional algorithm. The reverse algorithm using Mediace TPLA as a screening test is more sensitive for the detection of syphilis.
Go to : 
The diagnosis of syphilis is primarily based on serology, since the natural course of the infection is characterized by periods without clinical manifestations. Traditionally, the Center for Disease Control and Prevention (CDC) recommends a nontreponemal test, such as rapid plasma reagin (RPR), for syphilis serologic screening [1]. This algorithm is cost-effective and reliably correlates positive test results with the disease status. However, a nontreponemal test as a screening assay has significant limitations, including lack of specificity, manual operation, and subjective interpretation of results [2]. The availability of treponemal tests based on automatable enzyme- and chemiluminescence immunoassays (EIA/CIA) has reduced the time and labor required for the test. With the introduction of automated systems, treponemal screening is gaining acceptability, and its usefulness is justified [2].
Recently, the CDC has recommended a reverse algorithm, in which automated treponemal tests, such as EIA and CIA, are first performed as a screening test, followed by a nontreponemal test as a second step [3]. The CDC reported that the percentage of patients with false-positive EIA/CIA results in low-prevalence populations was especially high and 2.9-times greater than those in high-prevalence populations [3]. Accordingly, the implementation of the reverse algorithm has created a substantial amount of confusion and concern among healthcare providers and patients. Therefore, the CDC continues to recommend the traditional algorithm. The United Kingdom Health Protection Agency and the International Union Against Sexually Transmitted Infections encourage the use of a reverse algorithm that begins with a treponemal test [45].
In Korea, the cost of a treponemal test is paid by the National Health Insurance service only when it is performed as a second step following a nontreponemal test. Therefore, the nontreponemal test has been used for syphilis screening, except in health checkups. The Automated Mediace Treponema pallidum Latex Agglutination (TPLA; Sekisui Chemical, Osaka, Japan) and the Mediace rapid plasma reagin (RPR; Sekisui Chemical) are turbidoimmunoassays that are used by many laboratories in Korea and Japan. According to an external quality assessment program of the Korean Association of Quality Assurance for Clinical Laboratory, 23.3% of the institutes which performed the nontreponemal test used the turbidoimmunoassay, while 10.3% of the institutes which performed the treponemal test used the turbidoimmunoassay [6]. However, the Mediace RPR test has a low detection sensitivity for latent syphilis [7]. In this study, we compared the results of the traditional syphilis screening algorithm and the reverse algorithm with automated Mediace RPR or Mediace TPLA as first-line screening assays to evaluate their screening performance.
Go to : 
A total of 24,681 serum samples were obtained from subjects undergoing a health checkup between July 2007 and December 2012. Mediace RPR and Mediace TPLA were performed simultaneously for these samples. This study was approved by the institutional review board of Dongguk University Ilsan Hospital, Korea.
Mediace RPR is a latex turbidoimmunoassay using latex particles coated with lecithin and cardiolipin. Mediace TPLA is a latex turbidoimmunoassay using latex particles coated with T. pallidum antigens. The Hitachi 7600 automated chemistry analyzer (Hitachi, Tokyo, Japan) was used for the automated procedure and analysis. Results of the Mediace RPR ≥1.0 R.U. and Mediace TPLA ≥10.0 T.U. were considered positive.
Samples positive for syphilis according to one or both of the Mediace RPR and Mediace TPLA assays were stored at -70℃ after testing. Additional tests were performed on these samples according to the recommendations of the CDC reverse syphilis screening algorithms. Samples with discordant results (e.g., positive Mediace TPLA and negative Mediace RPR) were tested with T. pallidum particle agglutination (Fujirebio, Tokyo, Japan; TPPA) for confirmation. IgG immunoblot (Euroimmune, Luebeck, Germany) was performed as a supplementary confirmatory test in samples with discrepant Mediace TPLA and TPPA results (e.g., positive Mediace TPLA, negative Mediace RPR and negative TPPA). Only the Mediace TPLA and Mediace RPR results were reported to clinicians.
According to the traditional algorithm, samples with negative results by Mediace RPR screening were considered negative for syphilis. We reviewed the Mediace TPLA results on samples that tested positive using Mediace RPR. Samples tested positive by Mediace TPLA were considered positive for syphilis. Samples tested negative by Mediace TPLA were considered negative for syphilis (false positive results of screening test). According to the reverse algorithm, samples testing negative in Mediace TPLA screening were considered negative for syphilis. We reviewed the Mediace RPR result on samples tested positive by Mediace TPLA screening. Samples testing positive by Mediace RPR were considered positive for syphilis. Samples showing negative results by Mediace RPR and TPPA were considered negative for syphilis (false positive results of screening test). In case of a negative result for Mediace RPR and a positive result for TPPA, samples were considered positive for syphilis.
The following percentages were calculated for the traditional algorithm: the positive screening rate, the false-positive rate among all samples and among those with positive screening, and the identified syphilis rate among those with positive screening. The same percentages were also calculated for the reverse algorithm, and additionally, the false-positive rate and identified syphilis rate among discordant samples were calculated.
The medical charts of patients positive for nontreponemal and/or treponemal tests were reviewed to collect information regarding the previous treatment history for syphilis, start of syphilis treatment, and the clinical follow-up after the health checkup.
Go to : 
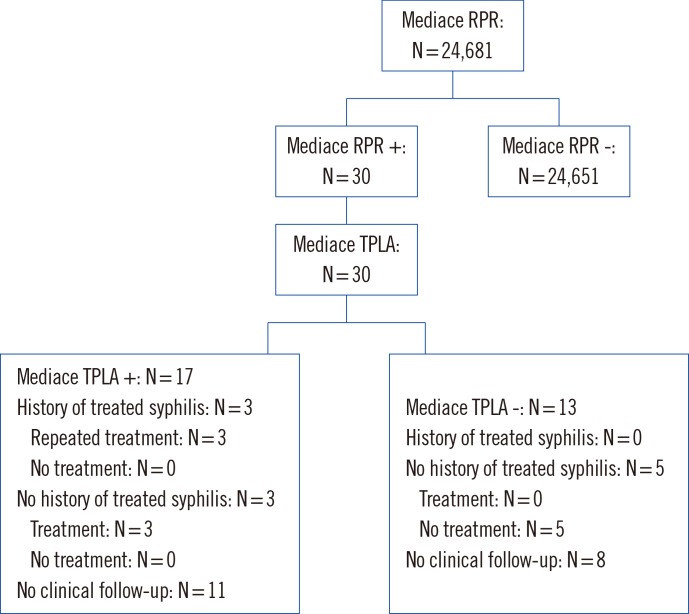
Of the 24,681 samples, 30 (0.1%) were found positive by using the traditional screening test. Of 30 Mediace RPR positive samples, 17 (56.7%) were confirmed as positive by Mediace TPLA and were suggestive of syphilis. Thirteen (43.3%) were found to be false positive by the traditional screening test. The identified syphilis rate was 0.07% (17/24,681) by the traditional algorithm, and the overall false positive rate was 0.05% (13/24,681).
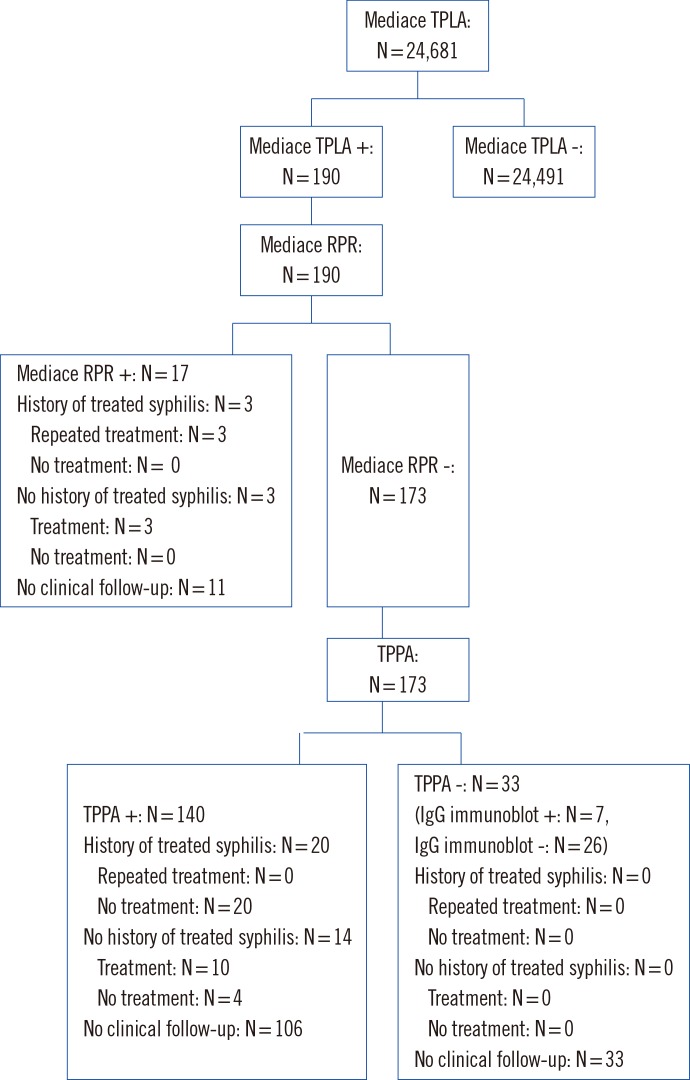
Of the 24,681 samples, 190 (0.8%) tested positive in the reverse screening test. Seventy (8.9%) of the 190 Mediace TPLA-positive samples were Mediace RPR-positive. Discordant samples (n=173) were tested with TPPA, and 140 (80.9%) were tested positive. Thirty three (19.1%) samples showed negative TPPA results, suggesting that the TPLA results for these samples were false positive. Among 33 samples showing different results between Mediace TPLA and TPPA, seven were confirmed as positive by IgG immunoblot. The identified syphilis rate was 0.64% (157/24,681) by the reverse algorithm. The overall false-positive rate was 0.13% (33/24,681).
Six patients with positive Mediace RPR and Mediace TPLA results according to the traditional algorithm (64.7% of 17 patients) had a clinical follow-up after the health checkup. All six received antibiotic treatment; three had no documentation of prior treatment for syphilis, and three had a prior treatment history (Fig. 1). Thirty four patients with Mediace TPLA-positive, Mediace RPR-negative, and TPPA-positive results by using the reverse algorithm (24.3% of 140 patients) underwent a clinical follow-up after the health checkup. Of the 34 patients, 20 with a history of treated syphilis did not receive antibiotic treatment. Of 14 patients (41.2% of 34 patients) without history of syphilis, 10 received antibiotic treatment (Fig. 2).
Go to : 
Several studies have evaluated the performance of automated Mediace TPLA and Mediace RPR. Comparative data demonstrated that the concordance of Mediace TPLA with the treponemal IgG/IgM immunoblot was higher than that of the Treponema pallidum hemagglutination assay (TPHA) and the treponemal IgG/IgM immunoblot [8]. The positive concordance rate between Mediace TPLA and the Architect Syphilis TP assay (Abbott Japan, Tokyo, Japan) was reported to be 96%, and the negative concordance rate was 97% [9]. We reported that the concordance rate between Mediace TPLA and the Architect Syphilis TP assay was 98% when tested with sera found positive by Mediace TPLA and related with false positivity of the treponemal test [10]. Therefore, it is clear that the Mediace TPLA analytical performance is valuable.
Mediace RPR performed by using samples of different stages of syphilis (primary, secondary, and latent stages) showed a sensitivity of 100%, 100%, and 82.9%, respectively [11]. Another study proposed that Mediace RPR could not be used as an alternative to the manual RPR test for a quantitative analysis of RPR titer because of the low correlation between the manual RPR and Mediace RPR (83.8%) results and the low detection rate of latent syphilis (55.6%) [12]. Despite the low sensitivity of Mediace RPR for latent syphilis, its use as a screening test has continued to increase because of its advantages of automation and applicability in many chemical analyzers.
The positive screening rate was reported as 1.5% by the reverse algorithm compared with 0.4% by the traditional algorithm in a low-prevalence population [13]. In another low-prevalence population, the positive screening rate was 4.0% by the reverse algorithm and 1.8% by the traditional algorithm [14]. The positive screening rate of syphilis in our study setting was lower than those reported for other low-prevalence setting studies. Moreover, in our study, the percentage of screening positivity by the reverse algorithm was eight times that of the traditional algorithm (0.8% vs. 0.1%), which is different from prior studies. In South Korea, the prevalence of syphilis is reported to be approximately 0.2% in the general population over 60 yr of age [15]. An equal positive screening rate of 0.05% was reported for Mediace RPR and Mediace TPLA in subjects undergoing health checkups [8]. In concordance with a previous study, the positive rate of syphilis was 0.07% by using the traditional algorithm. Therefore, it appears that our study was conducted on a very low-prevalence population in comparison with the previous study. However, the reason for this rate difference in traditional and reverse screening positivity cannot be explained by the study population alone. Other factors may include low sensitivity of Mediace RPR for latent syphilis and a high false-positive rate of reverse screening [1112].
Previous studies demonstrated a high percentage of false positives using EIA [3131416]. Reverse screening with EIA/CIA yields a higher false-positive rate than traditional testing does (0.6% vs. 0.0%) in a population with a low prevalence of syphilis [13]. A total of 334 out of 491 (68.0%) chemiluminescent microparticle immunoassay-reactive samples tested negative by using RPR, of which 137 (41.0%) also tested negative by TPPA in a low-prevalence population [14]. The CDC published that the discordant rate in low-prevalence and high-prevalence populations was 60.6% (1,807/2,984) and 50.6% (936/1,850), respectively. Of all discordant samples, 40.6% (737/1,807) of the low-prevalence population and 14.1% (1,297/936) of the high-prevalence population were also found negative by a second treponemal test [3]. Another study reported similar results: 439 (2%) out of 21,623 samples were CIA-positive, and 255 out of these 439 (58%) were RPR-negative. Subsequently, 71 of these 255 samples (28%) were negative on the basis of TPPA [17]. These data suggest that reverse screening may identify a higher number of patients with false-positive results than the traditional algorithm, especially if it is used in areas with a low prevalence [18]. In our study, the overall false-positive rate was 0.05% (13/24,681) according to the traditional algorithm and 0.13% (33/24,681) according to the reverse algorithm. These results are in agreement with previous studies. However, the present result differs from another study conducted in a low-prevalence population, where only 33 (19.1%) of 173 discordant samples had negative TPPA results. These results indicate a low false-positive rate of Mediace TPLA compared with previous EIA/CIA data. A different analytical level of treponemal tests for the implication of syphilis screening algorithms was reported [19]. Moreover, seven of 33 samples showing different results for TPLA and TPPA were confirmed as positive following IgG immunoblot.
Despite the overall increase of false-positive results compared with the traditional algorithm, the reverse algorithm using Mediace TPLA detected 140 samples with treponemal antibody that were not detected by the traditional algorithm. Mediace TPLA-positive, Mediace RPR-negative, and TPPA-positive results may occur in patients with latent syphilis or in those with prior successful treatment of syphilis [2]. Patients of the latter group can test positive by using Mediace TPLA and TPPA, which may lead to unnecessary follow-up and anxiety for the patient. Because patients made their decision for the clinical follow-up after the health checkup, we could not analyze all the clinical follow-up data. Thirty-four (24.3%) of 140 patients with Mediace TPLA-positive, Mediace RPR-negative, and TPPA-positive results by the reverse algorithm had a clinical follow-up after the health checkup. None of these patients had symptoms of primary or secondary syphilis. Fourteen of the 34 patients (41.2%) had no prior treatment for syphilis, but 10 of them were diagnosed with latent syphilis and received antibiotic treatment. Latent syphilis is defined as syphilis characterized by seroreactivity without other evidence of the disease. The CDC recommends treating patients diagnosed with latent syphilis [1]. The reverse algorithm was more sensitive than the traditional algorithm in detecting cases of latent syphilis, which were missed by the traditional screening algorithm. These results are similar to previous data suggesting that the missed-diagnosis rate is considerable (24.2%; 665 of 2,749 syphilis patients) with the traditional algorithm [20]. Another study reported that the reverse algorithm identified 21 RPR-negative syphilis patients in 792 samples [21].
In conclusion, although the problem of successfully treated patients showing positive results by the reverse algorithm still remains, the reverse algorithm using Mediace TPLA detected 140 samples with treponemal antibody that were not detected by the traditional algorithm using Mediace RPR. The reverse algorithm using Mediace TPLA as the screening test may enhance the sensitivity of syphilis diagnosis.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. Morbidity and Mortality Weekly Report (MMWR), Recommendations and reports. Centers for Disease Control and Prevention;2010. 59:p. 1–110.
2. Binnicker MJ. Which algorithm should be used to screen for syphilis? Curr Opin Infect Dis. 2012; 25:79–85. PMID: 22156894.

3. Centers for Disease Control and Prevention. Discordant results from reverse sequence syphilis screening--five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011; 60:133–137. PMID: 21307823.
4. Egglestone SI, Turner AJ. PHLS Syphilis Serology Working Group. Serological diagnosis of syphilis. Commun Dis Public Health. 2000; 3:158–162. PMID: 11014025.
5. French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H. IUST. IUSTI: 2008 European Guidelines on the Management of Syphilis. Int J STD AIDS. 2009; 20:300–309. PMID: 19386965.

6. External quality assurance immunoserology data. Updated on 2013. http://www.lab-qa.org/sub/catalog.php?CatNo=43&Mode=view&no=283.
7. Song EY, Yang JS, Chae SL, Kim S, Choi YS, Cha YJ. Current status of external quality assessment of syphilis test in Korea. Korean J Lab Med. 2008; 28:207–213. PMID: 18594173.

8. Huh HJ, Chae SL, Oh DJ, Park Q, Lim CS, Um TH, et al. Establishment and multicenter evaluation of a national reference panel for syphilis antibodies in Korea. Lab Med Online. 2014; 4:36–42.

9. Watanabe N, Nagatomo R, Okubo S, Yokota H, Ikeda H, Yatomi Y. Evaluation of latex agglutination test for anti-treponemal antibody in comparison with chemical luminescence tests. Rinsho Byori. 2011; 59:115–120. PMID: 21476292.
10. Huh HJ, Chae SL. Analysis of false positive rates and concordance rates in architect syphilis TP assay and Mediace TPLA, automated syphilis serologic tests. Dongguk J Med. 2009; 16:236–242.
11. Noh J, Ko HH, Yun Y, Choi YS, Lee SG, Shin S, et al. Evaluation of performance and false positivity of Mediace RPR test that uses a chemistry autoanalyzer. Korean J Lab Med. 2008; 28:312–318. PMID: 18728382.

12. Kim YS, Lee J, Lee HK, Kim H, Kwon HJ, Min KO, et al. Comparison of quantitative results among two automated Rapid Plasma Reagin (RPR) assays and a manual RPR test. Korean J Lab Med. 2009; 29:331–337. PMID: 19726896.

13. Binnicker MJ, Jespersen DJ, Rollins LO. Direct comparison of the traditional and reverse syphilis screening algorithms in a population with a low prevalence of syphilis. J Clin Microbiol. 2012; 50:148–150. PMID: 22090407.

14. Lipinsky D, Schreiber L, Kopel V, Shainberg B. Validation of reverse sequence screening for syphilis. J Clin Microbiol. 2012; 50:1501. PMID: 22259212.

15. Cho YH, Kim HO, Lee JB, Lee MG. Syphilis prevalence has rapidly decreased in South Korea. Sex Transm Infect. 2003; 79:323–324. PMID: 12902586.

16. Marangoni A, Moroni A, Accardo S, Cevenini R. Laboratory diagnosis of syphilis with automated immunoassays. J Clin Lab Anal. 2009; 23:1–6. PMID: 19140205.

17. Park IU, Chow JM, Bolan G, Stanley M, Shieh J, Schapiro JM. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J Infect Dis. 2011; 204:1297–1304. PMID: 21930610.

18. Loeffelholz MJ, Binnicker MJ. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol. 2012; 50:2–6. PMID: 22090405.
19. Jost H, Castro A, Cox D, Fakile Y, Kikkert S, Tun Y, et al. A comparison of the analytical level of agreement of nine treponemal assays for syphilis and possible implications for screening algorithms. BMJ Open. 2013; 3:e003347.

20. Tong ML, Lin LR, Liu LL, Zhang HL, Huang SJ, Chen YY, et al. Analysis of 3 algorithms for syphilis serodiagnosis and implications for clinical management. Clin Infect Dis. 2014; 58:1116–1124. PMID: 24550376.

21. González V, Fernández G, Dopico E, Margall N, Esperalba J, Muñoz C, et al. Evaluation of the Vitros Syphilis TPA chemiluminescence immunoassay as a first-line method for reverse syphilis screening. J Clin Microbiol. 2015; 53:1361–1364. PMID: 25609729.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download