Abstract
Carbapenemase-producing organisms (CPO) are rapidly disseminating worldwide, and their presence in tertiary care hospitals poses a significant threat to the management of nosocomial infections. There is a need to control CPO, especially in intensive care unit (ICU) patients, because these organisms are resistant to most β-lactam antibiotics and are easily transmitted. At present, the identification of CPO is time-consuming; hence, this study focused on the use of the Xpert CARBA-R assay (Cepheid, USA) to determine intestinal colonization rates of CPO in patients admitted to the ICU of a tertiary care hospital in Korea. Forty clinical stool samples were collected and inoculated both in a CARBA-R cartridge and in conventional culture plates. The CARBA-R assay required only ~one hour to screen CPO, while the time required for conventional culture was over three days. We also found that the prevalences of intestinal colonization by carbapenem-resistant organisms and Enterobacteriaceae were 17.5% (7 out of 40) and 7.5% (3 out of 40), respectively. Among the colonizing strains, three that contained carbapenemase, including Klebsiella pneumonia carbapenemase (KPC), and imipenem (IMP) and Verona integron-mediated metallo-β-lactamase (VIM) were found. With its convenience, the Xpert CARBA-R assay can be included in CPO surveillance strategies.
A rapid increase in the prevalence of carbapenem-resistant organisms (CRO) has been reported in Korea, particularly in tertiary care hospitals [12]. Among the different resistance mechanisms in CRO, the most important is the production of the carbapenemase enzyme. The reason for concern about these carbapenemase-producing organisms (CPO), which are a subset of CRO, is that these enzymes can induce high levels of resistance to most β-lactam antibiotics, including carbapenems, which are the last line of defense in the treatment of gram-negative Enterobacteriaceae infections. In addition, CPO can spread and have been related to outbreaks of carbapenem-resistant bacteria in both developed and developing countries [34567]. In Korea, six multidrug-resistant organisms, including CPO, have been implicated as the main agents of nosocomial infections according to the Korean government. It has been mandated for hospitals to report infections by these organisms to the Korean Center for Disease Control and Prevention since 2012 (http://www.cdc.go.kr/CDC/).
The risk factors for infection with multidrug-resistant organisms include a previous invasive procedure, diabetes mellitus, solid tumors, tracheostomy, urinary catheter insertions, and receipt of antipseudomonal penicillin [9]. Because the above factors are common in intensive care unit (ICU) patients, the detection of CRO or CPO colonization, which easily leads to true infections or to the horizontal transfer of carbapenem resistance determinants to other species [10], is very important for infection prevention. According to the study reported in 2012, the prevalence of fecal carriage of carbapenem-resistant Enterobacteriaceae (CRE) was 0.3%; however, to date, there have been no carbapenemase-producing Enterobacteriaceae (CPE) reported in the ICUs of Korean tertiary care hospitals [11]. Furthermore, a Chinese study in 2012 reported the prevalences of CRE and CPE, including Klebsiella pneumonia carbapenemase (KPC)-2, imipenem (IMP)-4, and New Delhi metallo-β-lactamase (NDM)-1 producers, to be 6.6% and 2.6%, respectively [12]. However, these previous studies utilized conventional methods, which were time-consuming and laborious. Recently, the Xpert CARBA-R assay (Cepheid, Sunnyvale, CA, USA) has been introduced for the detection of CPO from clinical samples. This assay is based on a multiplex real-time PCR technique and can detect blaVIM, blaIMP, blaNDM, blaKPC, and blaOXA-48-like alleles. However, reports on its clinical application have been scarce [13]. Hence, we applied the CARBA-R assay to determine the colonization rate of CPO in ICU patients in a tertiary care hospital in Korea and compared its results with those of conventional culture methods.
From July to August 2013, a total of 102 clinical samples were collected from ICU patients. Of these, the samples from patients in neonatal ICUs or patients with less than five-day stay in ICU were excluded. Additionally, duplicate samples from the same patients were excluded. In total, 40 clinical samples, including 23 stool samples and 17 rectal swabs, from 40 patients were evaluated. One of the rectal swabs was inoculated into the sample reagent and loaded into the cartridge of the CARBA-R assay according to the manufacturer's instructions, while another swab was inoculated in MacConkey broth containing 1 µg/mL meropenem (MEM) and incubated for 24 hr. The organisms from the broth containing MEM were then sub-cultured on MacConkey agar plates, each containing an MEM disk. Colonies growing near the MEM disks were sub-cultured on MacConkey agar containing 2 µg/mL MEM to ascertain resistance to carbapenem. Species were identified by using Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry (Bruker Daltonics, Bremen, Germany). For carbapenemase screening, a modified Hodge test using an IMP disk and a double disk synergy (DDS) test using aminophenyl boronic acid (APBA) and dipicolinic acid (DPA) vs. MEM were performed [14].
Three out of 40 samples (7.5%) tested positive by the CARBA-R assay, i.e., they showed one blaVIM-, one blaIMP-, and one blaKPC-positive signal, indicating that the prevalence of CPO was substantial in these ICU patients (Table 1). By conventional culture, 16 out of 40 samples showed bacterial growth close to the MEM disk on MacConkey agar. Of these, seven isolates (three Klebsiella pneumoniae, three Pseudomonas aeruginosa, and one Pseudomonas monteilii) were resistant to MEM with a minimum inhibitory concentration (MIC) over 2 µg/mL. Among the seven MEM-resistant strains, two K. pneumoniae and three P. aeruginosa were isolated from samples that tested negative by Xpert CARBA-R assay. For these five strains, the modified Hodge test was negative. In addition, enhanced inhibition zones were observed around APBA disks but not around DPA disks for four strains, and one strain showed no inhibition zone around either the APBA and DPA disks. This indicated that over-expression of Amp-C β-lactamase along with porin loss might be the causes of carbapenem resistance in these strains.
One P. monteilii isolate from a blaVIM-positive sample was negative for the modified Hodge test using an IPM disk but positive for the DDS test, indicating the limitations of the modified Hodge test in carbapenemase screening owing to its relatively low sensitivity [151617]. Additionally, no bacterial growth was observed from the sample showing a positive signal for blaIMP-, indicating that the CARBA-R assay is sensitive enough to detect CPO even in a rectal swab sample with a very low bacterial concentration. Alternatively, this might indicate a limitation of this study: we followed the MEM resistance cut-off value for Enterobacteriaceae in the CLSI guidelines [18]; however, this cut-off can cause low levels of CPO resistance to be missed. In addition, a recent report showed that the CARBA-R assay does not perform well in the detection of OXA-48-producing Escherichia coli [19]. Therefore, further studies are required to determine the prevalence of CPO in specific settings and to determine the accuracy of the CARBA-R assay with organisms that exhibit a low level of resistance to carbapenems.
Regarding the turn-around time, while over three days were required for the conventional culture, the CARBA-R assay required only about one hour, including 48 min running time.
The prevalences of intestinal colonization by CRO and CRE were 17.5% and 7.5%, respectively, in the ICUs of a tertiary care hospital in Korea, which is higher than those previously reported in similar settings [1112]. However, owing to the limited number of samples, further study will be needed to determine the true prevalence of CPO and CPE in the guts of ICU patients. The Xpert CARBA-R assay was found to be an easy-to-use assay, considering the labor and processing time required for conventional culture. The Xpert CARBA-R assay t should be adopted for surveillance and the determination of CPO colonization rates in clinical settings.
Acknowledgments
Cartridges for this study were provided by Cepheid. This work was supported by BioNano Health-Guard Research Center, funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea, as a Global Frontier Project (Grant Number H-GUARD_2014M3A6B2060509).
References
1. Yong D, Shin HB, Kim YK, Cho J, Lee WG, Ha GY, et al. Increase in the prevalence of carbapenem-resistant Acinetobacter isolates and ampicillin-resistant non-typhoidal Salmonella species in Korea: a KONSAR Study Conducted in 2011. Infect Chemother. 2014; 46:84–93. PMID: 25024870.
2. Chung HS, Lee Y, Park ES, Lee DS, Ha EJ, Kim M, et al. Characterization of the multidrug-resistant Acinetobacter species causing a nosocomial outbreak at intensive care units in a Korean Teaching Hospital: suggesting the correlations with the clinical and environmental samples, including respiratory tract-related instruments. Ann Clin Microbiol. 2014; 17:29–34.
3. Cornaglia G, Mazzariol A, Lauretti L, Rossolini GM, Fontana R. Hospital outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-1, a novel transferable metallo-beta-lactamase. Clin Infect Dis. 2000; 31:1119–1125. PMID: 11073738.
4. Gregory CJ, Llata E, Stine N, Gould C, Santiago LM, Vazquez GJ, et al. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect Control Hosp Epidemiol. 2010; 31:476–484. PMID: 20334553.
5. Woodford N, Tierno PM Jr, Young K, Tysall L, Palepou MF, Ward E, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob Agents Chemother. 2004; 48:4793–4799. PMID: 15561858.
6. Gibb AP, Tribuddharat C, Moore RA, Louie TJ, Krulicki W, Livermore DM, et al. Nosocomial outbreak of carbapenem-resistant Pseudomonas aeruginosa with a new bla(IMP) allele, bla(IMP-7). Antimicrob Agents Chemother. 2002; 46:255–258. PMID: 11751148.
7. Tokatlidou D, Tsivitanidou M, Pournaras S, Ikonomidis A, Tsakris A, Sofianou D. Outbreak caused by a multidrug-resistant Klebsiella pneumoniae clone carrying blaVIM-12 in a university hospital. J Clin Microbiol. 2008; 46:1005–1008. PMID: 18199780.
8. Wrenn C, O'Brien D, Keating D, Roche C, Rose L, Ronayne A, et al. Investigation of the first outbreak of OXA-48-producing Klebsiella pneumoniae in Ireland. J Hosp Infect. 2014; 87:41–46. PMID: 24746608.
9. Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012; 40:421–425. PMID: 21906844.
10. Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014; 20(Suppl 1):1–55. PMID: 24329732.

11. Kim J, Lee JY, Kim SI, Song W, Kim JS, Jung S, et al. Rates of fecal transmission of extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacteriaceae among patients in intensive care units in Korea. Ann Lab Med. 2014; 34:20–25. PMID: 24422191.
12. Zhao ZC, Xu XH, Liu MB, Wu J, Lin J, Li B. Fecal carriage of carbapenem-resistant Enterobacteriaceae in a Chinese university hospital. Am J Infect Control. 2014; 42:e61–e64. PMID: 24773806.
13. Tenover FC, Canton R, Kop J, Chan R, Ryan J, Weir F, et al. Detection of colonization by carbapenemase-producing Gram-negative Bacilli in patients by use of the Xpert MDRO assay. J Clin Microbiol. 2013; 51:3780–3787. PMID: 24006011.

14. Song W, Hong SG, Yong D, Jeong SH, Kim HS, Kim HS, et al. Combined use of the modified Hodge test and carbapenemase inhibition test for detection of carbapenemase-producing Enterobacteriaceae and metallo-β-lactamase-producing Pseudomonas spp. Ann Lab Med. 2015; 35:212–219. PMID: 25729723.
15. Lee W, Chung HS, Lee Y, Yong D, Jeong SH, Lee K, et al. Comparison of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry assay with conventional methods for detection of IMP-6, VIM-2, NDM-1, SIM-1, KPC-1, OXA-23, and OXA-51 carbapenemase-producing Acinetobacter spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2013; 77:227–230. PMID: 23993215.
16. Park YJ, Song W. Strategies for interpretive standards of β-lactams susceptibility testing and identification of extended-spectrum β-lactamases and carbapenemases in Enterobacteriaceae. Ann Clin Microbiol. 2013; 16:111–119.
17. Seah C, Low DE, Patel SN, Melano RG. Comparative evaluation of a chromogenic agar medium, the modified Hodge test, and a battery of meropenem-inhibitor discs for detection of carbapenemase activity in Enterobacteriaceae. J Clin Microbiol. 2011; 49:1965–1969. PMID: 21430097.
18. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility tsting. M100-S24. Wayne, PA: Clinical and Laboratory Standards Institues;2014.
19. Decousser JW, Poirel L, Desroches M, Jayol A, Denamur E, Nordmann P. Failure to detect carbapenem-resistant Escherichia coli producing OXA-48-like using the Xpert Carba-R assay. Clin Microbiol Infect. 2015; 21:e9–e10. PMID: 25682281.
Table 1
Summary of the results showing positivity in CARBA-R assay or conventional culture assay
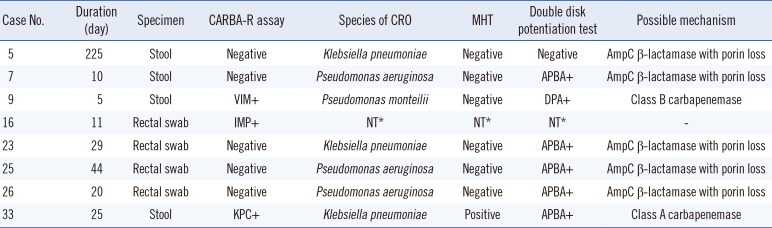
*Not tested owing to the lack of bacterial growth after overnight incubation in an enrichment (MacConkey) broth with 1 µg/mL MEM or no MEM-resistant colony around the MEM disk on MacConkey agar.
Abbreviations: APBA, aminophenylboronic acid; CCU, coronary care unit; CRO, carbapenem-resistant organism; DPA, dipicolinic acid; ERP, ertapenem; ICU, intensive care unit, IMP, imipenem; MEM, meropenem; MHT, modified Hodge test; NCU, neurosurgical care unit; NT, not tested; PCCU, pediatric critical care unit; VIM, Verona integron-mediated metallo-β-lactamase; KPC, Klebsiella pneumonia carbapenemase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download