Abstract
Background
We evaluated the reliability and accuracy of the combined use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) bacterial identification and Vitek 2 antimicrobial susceptibility testing (AST) for bacteria from positive blood culture bottles.
Methods
Direct identification and AST were performed in parallel to the standard methods in monomicrobial positive blood culture bottles. In total, 254 isolates grown on aerobic and/or anaerobic bottles were identified with MALDI-TOF Vitek MS (bioMérieux, France), and 1,978 microorganism/antimicrobial agent combinations were assessed. For isolates from anaerobic bottles, an aliquot of the culture broth was centrifuged, washed, and filtered through a nylon mesh. For isolates from aerobic/pediatric bottles, a lysis step using 9.26% ammonium chloride solution and 2% saponin solution was included.
Results
The overall correct identification rate was 81.8% (208/254) and that for gram-positive/gram-negative isolates was 73.9%/92.6%, respectively, and it was 81.8%, 87.6%, and 57.9% for isolates from aerobic, anaerobic, and pediatric bottles, respectively. Identification was not possible in 45 cases, and most of these isolates were streptococci (N=14) and coagulase-negative staphylococci (N=11). Misidentification occurred only in one case. Compared with standard methods, direct AST showed 97.9% (1,936/1,978) agreement with very major error of 0.25%, major error of 0.05%, and minor error of 1.8%.
Rapid identification and antimicrobial susceptibility testing (AST) of microorganisms causing bloodstream infections is pivotal to guide antimicrobial therapy [1]. Reducing the time before administration of appropriate antimicrobial therapy improves patient outcomes [2]. The current standard method for detecting causative agents of bloodstream infections is blood culture in commercially available blood culture systems using liquid medium [2]. When an automated blood culture system is used, signal-positive culture bottles are taken out of the system, and an aliquot of broth is subcultured onto solid media, which are incubated until visible colonies form, which takes up to two days [34].
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was recently introduced in the clinical microbiology laboratories for the identification of various microorganisms. Although it identifies bacteria and yeasts within a few minutes, it requires isolated colonies, which takes 18-48 hr of incubation [5]. To reduce the time needed for identification and AST, a few investigators tried to inoculate the microorganisms from positive blood culture bottles directly into several automated systems and MALDI-TOF MS systems [12356789101112]. However, direct identification from positive blood culture bottles requires sample preparation steps because these samples contain proteins/debris derived from human blood and culture broth, which could interfere with the spectra of the microorganisms [9]. Thus, lysis solutions (ammonium chloride, ethanol/formic acid/acetonitrile, and trifluoroacetic acid) [1291112], the lysis-filtration method [38], gel separation [5], or commercial kits [6] were used for sample preparation. However, some of these sample preparation procedures are laborious and/or expensive [11], and in the case of acetonitrile, samples must be prepared and manipulated in the fume hood because of acetonitrile's strong odor.
Only a few studies have been reported on the use of direct AST [237]. In combination with MALDI-TOF MS, the direct inoculation Vitek 2 AST method could yield results up to 24 hr earlier than the time taken by the standard method [3]. These studies have reported the effectiveness of direct identification of pathogens from positive blood culture bottles in combination with AST. However, these methods either used ethanol/formic acid/acetonitrile [2] or the labor-intensive lysis-filtration method [3], or evaluated only gram-negative isolates [7]. Therefore, in this study, we explored a simpler, faster, and more reliable protocol using an extraction method for direct identification and AST of bacteria from positive blood culture bottles.
In total, 254 blood cultures, obtained in the period from January to February 2015, were included in this study. Of these, 235 were samples obtained from adult patients, which included samples from two bottles (aerobic and anaerobic), and 19 were from pediatric patients. In parallel to the standard method, an aliquot from the positive blood culture bottle was taken for direct identification by Vitek MS (bioMérieux, Marcy L'Etoile, France) and full panel AST by Vitek 2 (bioMérieux). Results from direct identification and AST were not reported to the clinicians. This study was exempted by institutional review board of Seoul St. Mary's Hospital, Seoul, Korea.
Blood samples were collected and inoculated in blood culture bottles (Plus aerobic/F, Lytic/10 Anaerobic/F, Peds Plus/F; Becton Dickinson, Franklin Lakes, NJ, USA) and incubated in the BACTEC FX (Becton Dickinson) blood culture system. Signal-positive blood culture bottles were detected by continuous monitoring of CO2 level.
When a blood culture bottle was flagged as positive by the BACTEC system, an aliquot was taken from the positive blood culture bottle for Gram stain and subculture on solid media. Bottles showing more than one morphotype or yeasts on Gram stain were excluded from the study. Then, 100-µL aliquots were inoculated onto sheep blood agar (Asan BAP I; Asan Pharmaceutical Co., Ltd. Seoul, Korea) and MacConkey agar (Asan Mac II; Asan Pharmaceutical. Co., Ltd.). Agar plates were incubated in the dark in an atmosphere containing 5% CO2 at 37℃ for 24 hr. For anaerobic subculture, sheep blood agar was incubated at 37℃ in anaerobic atmosphere for two days. Colonies of the isolates formed on agar plates were used for identification and AST by the Vitek 2 system.
A standardized inoculum was prepared from the several colonies formed on agar medium, and the appropriate Vitek 2 cards were inoculated, following the manufacturer's recommendation. The GP, GN, ANC and NH cards (bioMérieux) were used for microbial identification, and the AST-P600, AST-P601, AST-ST01, AST-N224, and AST-N225 cards (bioMérieux) were used for AST of enterococci, staphylococci, streptococci, Enterobacteriaceae, and non-fermentative gram-negative bacteria (GNB), respectively. The resulting minimum inhibitory concentration (MIC) values were classified into clinical categories of susceptible, intermediate, or resistant following the CLSI guidelines [13].
The sample preparation method was different between anaerobic samples and aerobic/pediatric samples. As the anaerobic bottle contains the saponin ingredient (0.26% w/v), the lysis step was omitted. For samples from the anaerobic bottle, a 10-mL aliquot from positive blood culture bottles was centrifuged at 4,500g for 3 min, and the pellet was washed twice with 0.45% NaCl, suspended in 10 mL 0.45% NaCl, and filtered through a nylon mesh (66 µm pore size) to remove cell debris. The filtered sample was centrifuged at 4,500g for 3 min, and the pellet was suspended in 0.45% NaCl. The opacity of this suspension was adjusted to a 0.5 McFarland (McF) standard for direct AST by Vitek 2 and Gram stain. Samples showing mixed morphotypes or yeasts were excluded from the study.
For identification with Vitek MS, 100 µL of the suspension was centrifuged at 15,000g for 3 min. The pellet was mixed with 20 µL alcohol in the tube and placed on a target plate (Vitek MSDS, bioMérieux), dried at room temperature, and 1 µL of the CHCA matrix solution (α-cyano-4-hydroxycinnamic acid solution, bioMérieux) was applied to the spot. All isolates were tested once without duplication.
For aerobic/pediatric samples, to lyse red blood cells, a lysis step was included. Briefly, a 10-mL aliquot from a positive blood culture bottle was centrifuged at 4,500g for 3 min and lysis solution [9.26% ammonium chloride solution (0.1 mL) and 2% saponin solution (5 mL)] were added to the pellet, which was followed by vortexing for 10 sec and incubation for 10 min at room temperature. The resulting lysate was centrifuged at 4,500g for 3 min, washed twice with 0.45% NaCl, and processed as described above.
Mass spectra were obtained by using a Vitek MS system. The Vitek MS IVD system v2.0 was used for spectral analysis, and the results were compared with a reference library v2.0 of spectra provided by the manufacturer. The spectra were externally calibrated by using Escherichia coli (ATCC 8739). A bottle was considered to have a valid Vitek MS result if the target slide gave a confidence level ≥90%.
The identification was considered accurate, if the direct method by Vitek MS provided the same results as the standard method by Vitek 2 identification for any of the two bottles (aerobic/anaerobic). The identification was considered incorrect (misidentification), if Vitek MS provided results that were different from those of the standard method. Isolates were tagged as "no identification," if the Vitek MS database did not provide identification results. The warning messages "bad-spectrum" or "not enough peaks" appeared in case of a poor-quality deposit. For direct AST, the Vitek cards were selected according to the Gram stain results and direct identification results from the Vitek MS system. Comparison of AST between the direct and standard methods was expressed in terms of agreement, very major error (false susceptibility), major error (false resistance), or minor error (susceptible/resistance versus intermediate susceptibility).
Chi-square test or Fisher's exact test was used for statistical comparisons of the performance of aerobic/anaerobic bottles, as appropriate. A P value <0.05 was considered statistically significant.
Of 254 (146 gram-positive, 108 gram-negative) monomicrobial positive blood cultures evaluated, 233 isolates were identified to the species level and 21 isolates were identified to the genus level by the standard method using Vitek 2. Table 1 shows the identification results of the direct identification method using Vitek MS compared with those results of isolates grown using solid media culture and identified by Vitek 2. Overall, Vitek MS correctly identified 208 (81.8%) isolates, and 45 isolates were not identified. Misidentification occurred for only one sample; Propionibacterium acnes was misidentified as Clostridium bifermentans. Of the 208 correct identification results, 196 isolates (94.2%) were identified to the species level, and 12 isolates were identified to the genus level. According to the Gram stain results, Vitek MS correctly identified 108 (73.9%) of the gram-positive isolates and 100 (92.6%) of the gram-negative isolates. The proportion of correct identification for Enterobacteriaceae, non-fermentative GNB, and staphylococci was 81/83 (97.6%), 12/15 (80.0%), and 72/85 (84.7%), respectively.
Vitek MS did not provide identification results for 45 isolates, and among them, 29 samples were not identified owing to the "bad spectrum" warning message, nine samples were scored as "not-identified," and seven samples were not identified owing to the "not enough peaks" warning message. Most of these isolates were gram-positive (N=37); 14 streptococci, 11 coagulase-negative staphylococci (CNS), three enterococci, two Staphylococcus aureus, two Micrococcus spp., two Bacillus spp., and one each of Actinomyces odontolyticus, Finegoldia magna, and Peptostreptococcus spp.
Table 2 shows identification results according to the type of culture bottle. Of the 235 sets of positive blood culture bottles from adult patients, 182 aerobic bottles and 145 anaerobic bottles were flagged as positive and identified by the standard method by Vitek 2. No identification discordance was observed between the aerobic and anaerobic bottles from the same patient. The correct identification rate by the direct method was 149/182 (81.8%) and 127/145 (87.6%) for aerobic and anaerobic bottles, respectively (P=0.742). The correct identification rate of gram-positive isolates in aerobic and anaerobic bottles was 76.2% and 77.4%, respectively (P=0.960). The correct identification rate of gram-negative isolates in aerobic and anaerobic bottles was 88.8% and 97.3%, respectively (P=0.783). No identification was observed in 33/182 aerobic bottles and 17/145 anaerobic bottles (P=0.221; Table 2). In addition, 53 aerobic bottles and 90 anaerobic bottles were tagged as "no growth." Moreover, 90 isolates and 53 isolates were detected only in aerobic and anaerobic bottles, respectively. However, the rate of no identification among these 90 and 53 isolates was similar (23/90 isolates; 25.5%, 13/53 isolates; 24.5%). The disproportionate growth did not affect the identification rate between the bottles. Of the 19 pediatric bottles, 11 were correctly identified (five Staphylococcus epidermidis, one each of Staphylococcus aureus, Staphylococcus saprophyticus, Escherichia coli, Pseudomonas aeruginosa, Neisseria spp., and Micrococcus spp.), and eight were not identified (2 Staphylococcus capitis, 2 Staphylococcus epidermidis, and one each of Streptococcus pneumoniae, Micrococcus spp., Streptococcus constellatus, and Burkholderia cepacia).
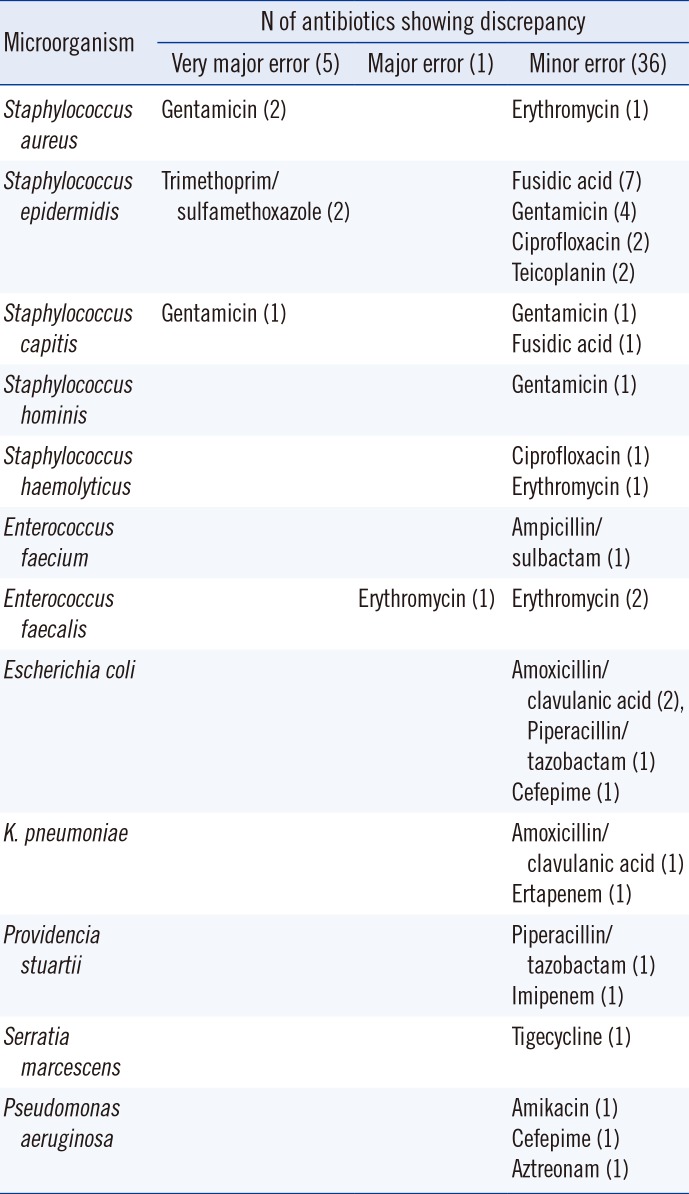
In total, 120 isolates (69 gram-positive cocci, 51 gram-negative bacteria) were analyzed for full panel AST by Vitek 2. Then, 1,978 isolate/antimicrobial agent combinations were analyzed. The direct AST results showed 97.9% (1,936/1,978) agreement with the standard method AST results. The rates for very major error, major error, and minor error were 0.25% (5/1,978), 0.05% (1/1,978), and 1.8% (36/1,978), respectively (Table 1). The microorganism/antimicrobial agent combinations that did not agree with standard methods are listed in Table 3.
Gram-positive isolates showed an agreement rate of 97.2% (1,051/1,081), with very major error rate of 0.5% (5/1,081), major error rate of 0.1% (1/1,081), and minor error rate of 2.2% (24/1,081). Staphylococcus epidermidis was the major cause of the disagreement, and gentamicin (N=9) and fusidic acid (N=8) were an attribute of high error rates. Among the gram-negative isolates, the overall agreement rate was 98.6% (885/897), with a minor error rate of 1.4% (12/897). Escherichia coli and Pseudomonas aeruginosa were the major causes of the disagreement, and amoxicillin/clavulanic acid (N=3) were an attribute of high error rates.
In this study, we evaluated the reliability and accuracy of performing direct inoculation of organisms from positive blood culture bottles in combination with Vitek MS and Vitek 2 to achieve rapid identification and AST. During the study period, only one sample containing yeast was identified, and we did not include this case in our study. The proportion of correct identification for Enterobacteriaceae, non-fermentative GNB, and staphylococci was 97.6%, 80.0%, and 84.7%, respectively. Gram-positive isolates showed a relatively low correct identification rate in other specimens, by the direct MALDI-TOF method [14]. Compared with the standard method, the direct method showed rapid and reliable results, especially for the gram-negative group. These data coincide with previous data that presented a better identification of gram-negative isolates (84-99%) when compared with gram-positive isolates (64.8-86.3%) [1561112].
Excluding the "no identification" results, the concordance rate of identification between the direct method and the standard method was very reliable (99.5%, 207/208), and most of the "no identification" results were attributed to the poor quality of the spots (which led to the appearance of "bad spectrum" or "not enough peaks" warning messages on the system). This method has limitations for the identification of CNS and streptococci; however, "no identification" results were obtained for these organisms, and they were not identified incorrectly. Other protocols that tried direct MALDI-TOF MS inoculation showed similar results (63.3-83.5%) for the identification of CNS isolates [12561112]. Prod'hom et al. [1] suggested that the lower yield of MALDI-TOF MS results with streptococci and staphylococci may be attributed to inter-species relatedness and bacterial cell wall composition. It is notable that misidentification occurred only for Propionibacterium acnes, which is known to be identified inaccurately with MALDI-TOF MS, even with the use of colonies [15].
Anaerobic bottles showed a higher correct identification rate than did aerobic bottles; however, the difference was not statistically significant. Given that sample preparation is simpler with anaerobic bottles, if the organism growth is observed using both aerobic and anaerobic bottles, we recommend using anaerobic bottles. Our simple sample preparation method resulted in a higher correct identification rate than that of the previous study (82.2%), which used centrifugation alone [7]. The relatively lower correct identification rate in pediatric bottles was mainly attributed to gram-positive isolates.
In the present study, the accuracy of AST was excellent with both gram-positive and gram-negative isolates, and the antimicrobial agreement rate was comparable or superior to that of other studies [237]. The relatively higher very major error rate among gram-negative bacteria observed in the study by Machen et al. [3] was observed with Proteus spp. against various antibiotics (ampicillin, cefazolin, ceftazidime, and ceftriaxone); however, in this study, Proteus spp. was not included. Very major error was found with Staphylococcus spp. alone, and it was against gentamicin and trimethoprim/sulfamethoxazole, which was also reported by Machen et al. [3].
In summary, our simple and cost-effective sample preparation method would be very useful for rapid identification and AST of bacteria from positive blood culture bottles. However, for identification of streptococci and CNS, further improvement in the method is needed.
Acknowledgments
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI12C1093) and also by the Technology Innovation Program (No: 10049771, Development of Highly-Specialized Platform for IVD Medical Devices) funded by the Ministry of Trade, Industry & Energy (MI, Korea).
References
1. Prod'hom G, Bizzini A, Durussel C, Bille J, Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J Clin Microbiol. 2010; 48:1481–1483. PMID: 20164269.
2. Romero-Gómez MP, Gómez-Gil R, Paño-Pardo JR, Mingorance J. Identification and susceptibility testing of microorganism by direct inoculation from positive blood culture bottles by combining MALDI-TOF and Vitek-2 Compact is rapid and effective. J Infect. 2012; 65:513–520. PMID: 22940580.

3. Machen A, Drake T, Wang YF. Same day identification and full panel antimicrobial susceptibility testing of bacteria from positive blood culture bottles made possible by a combined lysis-filtration method with MALDI-TOF VITEK mass spectrometry and the VITEK2 system. PLoS One. 2014; 9:e87870. PMID: 24551067.

4. Schneiderhan W, Grundt A, Wörner S, Findeisen P, Neumaier M. Work flow analysis of around-the-clock processing of blood culture samples and integrated MALDI-TOF mass spectrometry analysis for the diagnosis of bloodstream infections. Clin Chem. 2013; 59:1649–1656. PMID: 23881934.

5. Stevenson LG, Drake SK, Murray PR. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2009; 48:444–447. PMID: 19955282.

6. Kok J, Thomas LC, Olma T, Chen SC, Iredell JR. Identification of bacteria in blood culture broths using matrix-assisted laser desorption-ionization Sepsityper™ and time of flight mass spectrometry. PLoS One. 2011; 6:e23285. PMID: 21858058.

7. Ling TK, Liu ZK, Cheng AF. Evaluation of the VITEK 2 system for rapid direct identification and susceptibility testing of gram-negative bacilli from positive blood cultures. J Clin Microbiol. 2003; 41:4705–4707. PMID: 14532207.

8. Fothergill A, Kasinathan V, Hyman J, Walsh J, Drake T, Wang YF. Rapid identification of bacteria and yeasts from positive-blood-culture bottles by using a lysis-filtration method and matrix-assisted laser desorption ionization-time of flight mass spectrum analysis with the SARAMIS database. J Clin Microbiol. 2013; 51:805–809. PMID: 23254131.

9. Lavergne RA, Chauvin P, Valentin A, Fillaux J, Roques-Malecaze C, Arnaud S, et al. An extraction method of positive blood cultures for direct identification of Candida species by Vitek MS matrix-assisted laser desorption ionization time of flight mass spectrometry. Med Mycol. 2013; 51:652–656. PMID: 23373445.
10. Ferreira L, Sánchez-Juanes F, Porras-Guerra I, García-García MI, García-Sánchez JE, González-Buitrago JM, et al. Microorganisms direct identification from blood culture by matrix-assisted laser desorption/ionization time-of flight mass spectrometry. Clin Microbiol Infect. 2011; 17:546–551. PMID: 20456452.
11. Monteiro J, Inoue FM, Lobo AP, Sugawara EK, Boaretti FM, Tufik S. Fast and reliable bacterial identification direct from positive blood culture using a new TFA sample preparation protocol and the Vitek®MS system. J Microbiol Methods. 2015; 109:157–159. PMID: 25541363.
12. Marinach-Patrice C, Fekkar A, Atanasova R, Gomes J, Djamdjian L, Brossas JY, et al. Rapid species diagnosis for invasive candidiasis using mass spectrometry. PLos One. 2010; 5:e8862. PMID: 20111603.

13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 24th Informational supplement, M100-S24. Wayne, PA: National Committee for Clinical Laboratory Standards;2014.
14. Kim Y, Park KG, Lee K, Park YJ. Direct identification of urinary tract pathogens from urine samples using the Vitek MS system based on Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. Ann Lab Med. 2015; 35:416–422. PMID: 26131413.

15. Coltella L, Mancinelli L, Onori M, Lucignano B, Menichella D, Sorge R, et al. Advancement in the routine identification of anaerobic bacteria by MALDI-TOF mass spectrometry. Eur J Clin Microbiol Infect Dis. 2013; 32:1183–1192. PMID: 23584672.

Table 1
Comparison of identification and antimicrobial susceptibility testing results between the direct method and the standard method
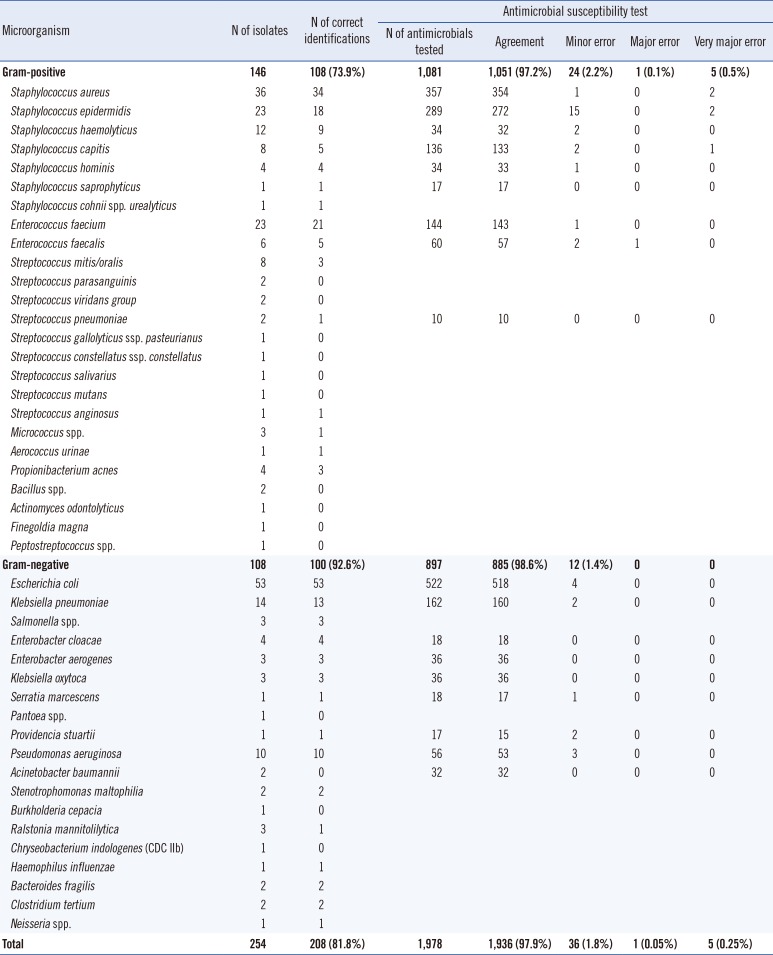
Table 2
Identification rates according to the type of blood culture bottle
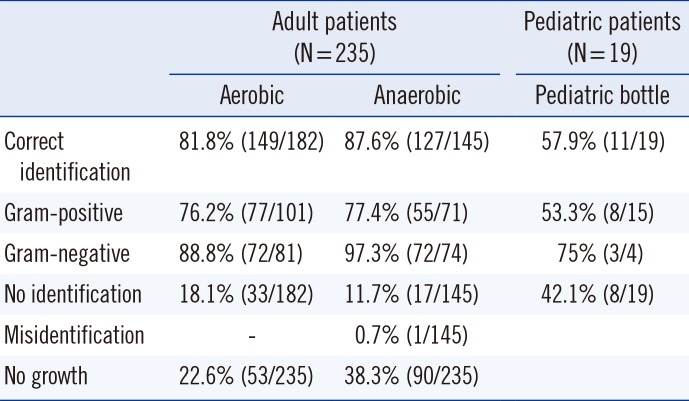
Table 3
Microorganism/antimicrobial agent combinations showing discrepancy between the results by the direct method and those by the standard method in antimicrobial susceptibility test by Vitek 2




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download