Abstract
Background
Burn wounds lack normal barriers that protect against pathogenic bacteria, and burn patients are easily colonized and infected by Staphylococcus aureus. Toxic shock syndrome (TSS) is a rare but fatal disease caused by S. aureus. A lack of detectable antibodies to TSS toxin-1 (TSST-1) in serum indicates susceptibility to TSS.
Methods
A total of 207 patients (169 men and 38 women; median age, 42.5 yr) admitted to a burn center in Korea were enrolled in this study. The serum antibody titer to TSST-1 was measured by sandwich ELISA. S. aureus isolates from the patients' nasal swab culture were tested for TSST-1 toxin production by PCR-based detection of the TSST-1 toxin gene.
Results
One hundred seventy-four (84.1%) patients showed positive results for antibody against TSST-1. All patients aged ≥61 yr (n=28) and <26 months (n=7) were positive for the anti-TSST-1 antibody. S. aureus was isolated from 70 patients (33.8%), and 58.6% of the isolates were methicillin resistant. Seventeen patients were colonized with TSST-1-producing S. aureus. The antibody positivity in these 17 carriers was 88.2%, and the positivity in the non-carriers was 83.7%.
Burn patients are susceptible to invasive infections, and complications from infection are a leading cause of burn-related morbidity and mortality [1, 2]. Staphylococcus aureus is the most frequent cause of infection in burn patients [3, 4]. Some S. aureus strains produce a variety of exotoxins such as toxic shock syndrome toxin-1 (TSST-1), staphylococcal enterotoxins, and exfoliative toxin [5], which increase the morbidity and mortality via systemic pathways that can induce shock and cause host immune disruption [2, 6].
Toxic shock syndrome (TSS) is an acute febrile illness caused by S. aureus and is characterized by fever, rashes, desquamation, hypotension, and multi-organ involvement [6, 7]. There are several toxins associated with staphylococcal TSS, but the major cause is TSST-1 [7, 8]. Reduced levels of serum antibody to TSST-1 are correlated with TSS development [9]. Many reports have shown that the prevalence of this antibody increases with age, and a majority of the adult population has already developed antibodies to TSST-1 [10, 11, 12]. Among patients with menstrual TSS, low or negative concentrations of such antibodies have been reported in 90.5% of patients, and more than 50% of these patients failed to seroconvert within 2 months of acquiring the infection [9]. TSS caused by S. aureus has rarely been reported; to our knowledge, thus far, only one case of TSS caused by methicillin-resistant S. aureus (MRSA) harboring TSST-1 gene has been reported in a burn patient from Korea [13]. In addition, the presence of the anti-TSST-1 antibody has not yet been characterized in the Korean population.
In this study, we evaluated the prevalence of the anti-TSST-1 antibody and nasal colonization of TSST-1-producing S. aureus among patients admitted to a burn center.
A total of 207 patients (169 men and 38 women; median age, 42.5 yr [range, 10 months to 87 yr]) admitted to the burn center of Hangang Sacred Heart Hospital, Seoul, Korea, from April through November 2009 were enrolled in the study. None of the patients had TSS before or during the hospital stay.
Serum and nasal swab samples were collected within 7 days of admission. The patients' sera were stored at -70℃ for analysis by ELISA, and nasal swabs were streaked onto mannitol salt agar plates for S. aureus screening.
The study protocol, informed consent, and other associated documents were reviewed and approved by the Institutional Review Board of Hangang Sacred Heart Hospital.
Serum antibody titers to TSST-1 were measured by sandwich ELISA, according to the method of Parsonnet et al. [11] with minor modifications. In brief, serum samples were serially diluted from 1:2 to 1:4,096 with phosphate-buffered saline and poured into wells of a microtiter plate precoated with TSST-1 (Sigma-Aldrich, St. Louis, MO, USA). Each plate was treated with goat anti-human IgG-horseradish peroxidase (MP Biomedicals, Solin, OH, USA) and subsequently with the substrate 3,3',5,5'-tetramethylbenzidine. The enzyme reaction was terminated by addition of 100 µL of 2M H2SO4 solution when the positive control wells almost reached an optical density of 1.0 at 405 nm. Commercially available human immunoglobulin G (I.V.-Globulin S inj.; Green Cross, Cheongju, Korea), diluted to 1:1,024 was arbitrarily used as a positive control, and a serum aliquot from a healthy volunteer was used as a titer control (1:16 dilution) in each ELISA for ensuring quality control. Samples with titers ≥1:16 were considered positive and those with titers ≤1:2 were considered negative. Titers of 1:4 and 1:8 were considered intermediate.
We selected 2 or 3 suspected colonies from the mannitol salt agar plates for identification of S. aureus. Tests for identification of and susceptibility to S. aureus isolates were performed by using Microscan (Siemens, West Sacramento, CA, USA). PCR was performed to detect the TSST-1 gene [14].
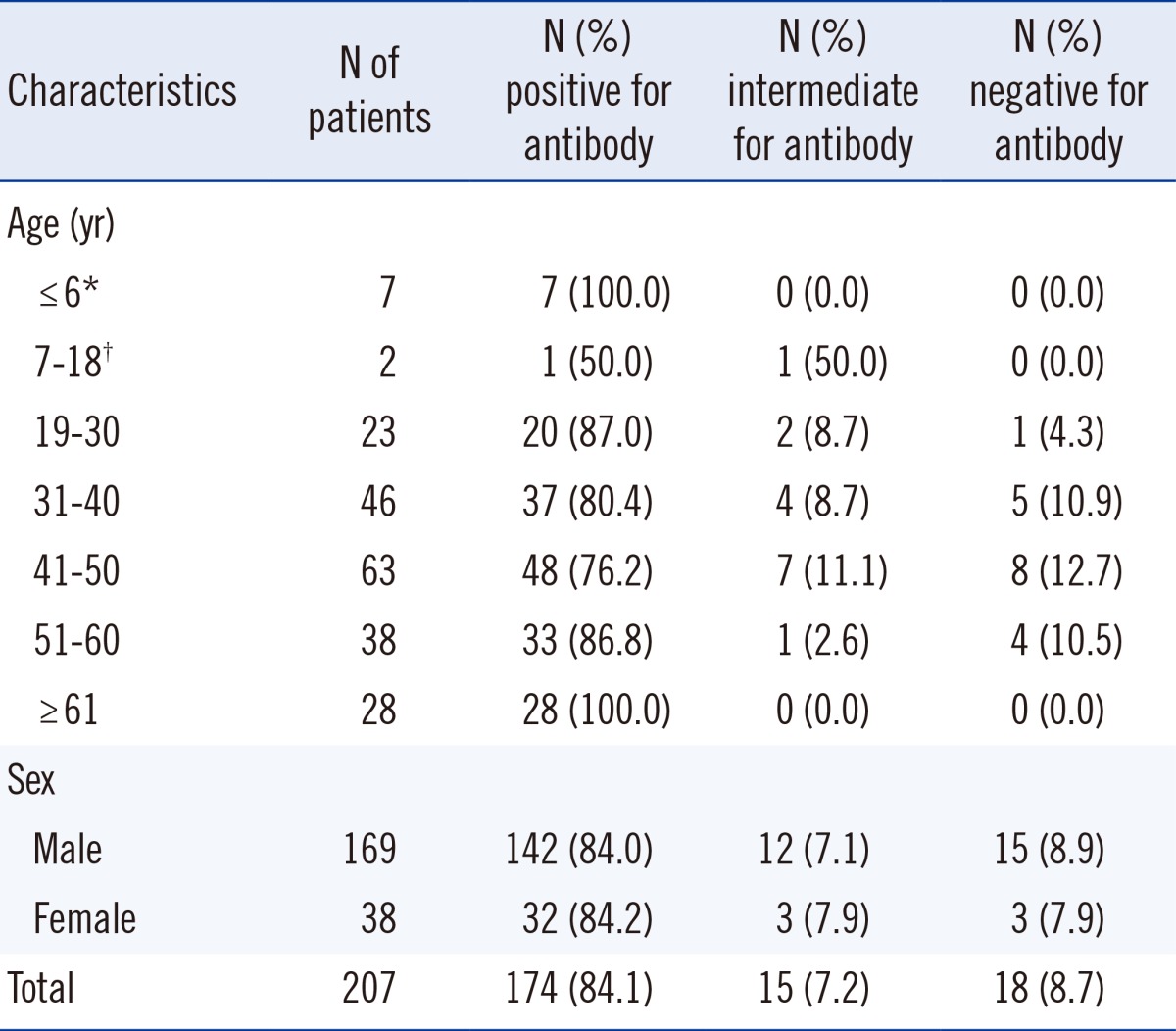
Among the 207 patients, 174 (84.1%) had positive titers of antibody to TSST-1 (≥1:16) and 18 (8.7%) had negative titers (≤1:2). All patients aged ≥61 yr (n=28) and <26 months of age (n=7) had positive titers of anti-TSST-1 antibody. No difference in the antibody prevalence was observed between men and women (84.0% and 84.2%, respectively) (Table 1).
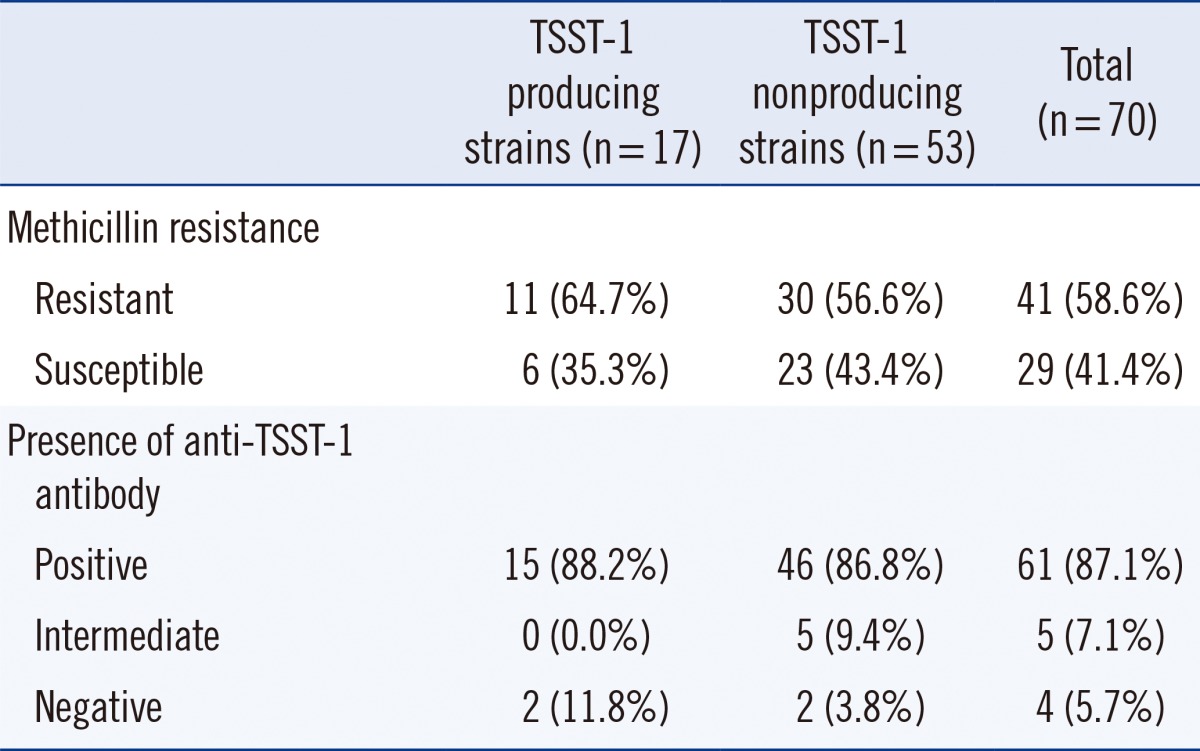
Of the 207 patients, 70 (33.8%) were colonized with S. aureus, and among them, 41 (58.6%) were MRSA carriers. Seventeen patients (8.2%; 24.3% of S. aureus carriers) were colonized with TSST-1-producing S. aureus; 11 isolates (64.7%) were identified as MRSA. Fifteen TSST-1-producing S. aureus carriers (88.2%) had positive titers for the anti-TSST-1 antibody (Table 2). Among the TSST-1-producing S. aureus carriers (n=17), all patients with methicillin-susceptible S. aureus (MSSA) colonization (n=6) were positive for the anti-TSST-1 antibody, and 5 of them had high titers of anti-TSST-1 antibody (≥1:512), while the MRSA carriers (n=11) had a lower positive titer for the anti-TSST-1 antibody (median titer, 1:1,536 vs. 1:32). Among the TSST-1-producing MRSA carriers (n=11), 2 patients were negative for the antibody, and only one patient had a high antibody titer (1:4,096).
The antibody positivity in the group of patients colonized with TSST-1-producing S. aureus was 88.2% (15/17) and that in the patients without TSST-1-producing S. aureus was 83.7% (159/190) (P>0.05). Additionally, patients with antibody titers ≥1:2,048 were frequently found to be colonized with the TSST-1-producing strain (Fig. 1).
TSS is a life-threatening condition, wherein superantigen-mediated activation of T cells results in overproduction of cytokines, resulting in systemic inflammation and shock [6, 15]. Although TSS appears to be a rare disease, the severity of superantigen-mediated disease is underestimated [7].
Burn wounds lack normal barriers that protect against pathogenic bacteria including S. aureus, and in many burn patients with nasal carriage of S. aureus at admission, subsequent colonization of burn wounds by identical strains is seen [16]. A lack of detectable antibodies to TSST-1 in serum indicates susceptibility to TSS [17, 18].
Nasal carriage rates of S. aureus might vary according to the study population and location. Park et al. [19] reported that the S. aureus nasal carriage rates among healthy students and healthcare workers of a university hospital in Korea were 30.0% and 42.2%, respectively, and the methicillin-resistance rates among S. aureus strains were 5.6% and 56.0%, respectively. Our study on burn patients showed an S. aureus nasal carriage rate of 33.8% (70/207), with a higher methicillin-resistant rate of 58.6% (41/70). MRSA carriage rate in hemodialysis patients in 7 Korean hospitals was reported to be 6.7-19.0% [20], whereas the rate in our study was 19.8% (41/207).
Although a previous report from Japan reported higher titers of TSST-1 antibody in the first 6 months after birth and subsequent lower titers up to the age of 2 yr [10], we found that all patients aged 10-26 months were positive for the anti-TSST-1 antibody. In addition, most burn patients (84.1%) had an antibody to TSST-1. This prevalence is comparable with that in Caucasian (86%) or Asian (84%) menstruating women in North America [11]. However, a much lower prevalence (47%) has been reported in healthy Japanese women [12], while another study reported 96% positivity in Japanese adults [10]. Antibody positivity was 100% in the elderly (≥61 yr), suggesting that the risk of TSS is low in the elderly. This finding is comparable with a previous report from Japan, in which the positivity for the antibody in persons aged >41 yr was 100% [10].
Nasal colonization with TSST-1-producing S. aureus was found in 8.2% of patients in the current study. The incidence was higher than that of healthy women living in North America (6%) or Japan (3%) [11, 12]. Peck et al. [21] reported 52.6% prevalence rate of TSST-1-producing strains in S. aureus isolates from nasal swabs of children attending an outpatient clinic in a Korean tertiary-care hospital; this rate is higher than ours (24.3%). In addition, they found no difference in the prevalence of TSST-1-producing strains between MRSA and MSSA strains (50.0% vs. 53.3%). In our study, the TSST-1-producing isolates accounted for 26.8% (11/41) of MRSA strains and 20.7% (6/29) of MSSA strains (P>0.05). On the contrary, in healthy Japanese women, the carriage rate of TSST-1-producing isolates was 100% (2/2) for MRSA strains and 6.5% (10/155) for MSSA strains [12].
Nasal carriers of TSST-1-producing S. aureus tended to have a high rate of antibody positivity, which is known to be associated with a lower risk of TSS. In the TSST-1-positive group, MRSA carriers had lower antibody levels. Because MRSA is less prevalent in the community, many MRSA carriers might be colonized with these strains after admission to the hospital. A TSST-1-producing MRSA carrier with the highest antibody titer (1:4,096) was referred to us from another hospital, and his total duration of hospital stay was 15 days, which is a sufficient period to generate antibodies to hospital-acquired MRSA.
In our study, an antibody titer ≥1:2,048 was more frequently observed in patients with colonization of the TSST-1-producing strain than in those without the toxigenic strain (23.5% vs. 2.1%). This finding is compatible with those of previous studies on menstruating women colonized with TSST-1-producing S. aureus [11, 12].
In summary, most patients (84.1%) admitted to a burn center in Korea had serum antibody to TSST-1. Elderly patients aged ≥61 yr and children aged <26 months had 100% prevalence of the antibody to TSST-1, while a few patients (~10%) aged 31-60 yr showed a negative antibody titer to TSST-1. Most of the burn patients with TSST-1-producing S. aureus had a positive titer of anti-TSST-1 antibody, and some patients had a negative titer of anti-TSST-1 antibody; such patients might be at risk for developing TSS.
Acknowledgments
This study was supported by the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (grant A084589).
References
2. Shupp JW, Ortiz RT, Moffatt LT, Jo DY, Randad PR, Njimoluh KL, et al. Treatment with an oxazolidinone antibiotic inhibits toxic shock syndrome toxin-1 production in MRSA-infected burn wounds. J Burn Care Res. 2013; 34:267–273. PMID: 23370994.

3. Lee HG, Jang J, Choi JE, Chung DC, Han JW, Woo H, et al. Bloodstream infections in patients in the burn intensive care unit. Infect Chemother. 2013; 45:194–201. PMID: 24265967.
4. Murray CK, Holmes RL, Ellis MW, Mende K, Wolf SE, McDougal LK, et al. Twenty-five year epidemiology of invasive methicillin-resistant Staphylococcus aureus (MRSA) isolates recovered at a burn center. Burns. 2009; 35:1112–1117. PMID: 19477601.
5. Marples RR, Wieneke AA. Enterotoxins and toxic-shock syndrome toxin-1 in non enteric staphylococcal disease. Epidemiol Infect. 1993; 110:477–488. PMID: 8519313.
6. McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001; 55:77–104. PMID: 11544350.

7. DeVries AS, Lesher L, Schlievert PM, Rogers T, Villaume LG, Danila R, et al. Staphylococcal toxic shock syndrome 2000-2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011; 6:e22997. PMID: 21860665.

8. Andrews MM, Parent EM, Barry M, Parsonnet J. Recurrent nonmenstrual toxic shock syndrome: clinical manifestations, diagnosis, and treatment. Clin Infect Dis. 2001; 32:1470–1479. PMID: 11317249.

9. Lappin E, Ferguson AJ. Gram-positive toxic shock syndrome. Lancet Infect Dis. 2009; 9:281–290. PMID: 19393958.
10. Quan L, Morita R, Kawakami S. Toxic shock syndrome toxin-1 (TSST-1) antibody levels in Japanese children. Burns. 2010; 36:716–721. PMID: 20036064.

11. Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol. 2005; 43:4628–4634. PMID: 16145118.
12. Parsonnet J, Goering RV, Hansmann MA, Jones MB, Ohtagaki K, Davis CC, et al. Prevalence of toxic shock syndrome toxin 1 (TSST-1)-producing strains of Staphylococcus aureus and antibody to TSST-1 among healthy Japanese women. J Clin Microbiol. 2008; 46:2731–2738. PMID: 18550735.
13. Choi JH, Choi JH, Kim DI, Kim JS, Choi EH. A case of toxic shock syndrome caused by methicillin-resistant Staphylococcus aureus (MRSA) following a burn injury. Korean J Pediatr Infect Dis. 2009; 16:205–209.
14. Mehrotra M, Wang G, Johnson WM. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol. 2000; 38:1032–1035. PMID: 10698991.
15. Xu SX, McCormick JK. Staphylococcal superantigens in colonization and disease. Front Cell Infect Microbiol. 2012; 2:52. PMID: 22919643.

16. Kooistra-Smid M, Nieuwenhuis M, van Belkum A, Verbrugh H. The role of nasal carriage in Staphylococcus aureus burn wound colonization. FEMS Immunol Med Microbiol. 2009; 57:1–13. PMID: 19486150.
17. Bonventre PF, Linnemann C, Weckbach LS, Staneck JL, Buncher CR, Vigdorth E, et al. Antibody responses to toxic-shock-syndrome (TSS) toxinby patients with TSS and by healthy staphylococcal carriers. J Infect Dis. 1984; 150:662–666. PMID: 6491377.
18. Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000; 13:16–34. PMID: 10627489.
19. Park BG, Lee MK. Nasal carriage of methicillin-resistant Staphylococcus aureus among healthcare workers and community students in 1997 and 2006. Korean J Nosocomial Infect Control. 2007; 12:85–90.
20. Kim JS, Lee SH, Jeong J, Roh KH, Lee HK, Jang SJ, et al. Nasal colonization and molecular characterization of methicillin-resistant Staphylococcus aureus among hemodialysis patients in 7 Korean hospitals. Korean J Nosocomial Infect Control. 2013; 18:51–56.
21. Peck KR, Baeck JY, Song JH, Ko KS. Comparison of genotypes and enterotoxin genes between Staphylococcus aureus isolated from blood and nasal colonizers in a Korean hospital. J Korean Med Sci. 2009; 24:585–591. PMID: 19654937.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download