Abstract
Background
Recently, the iNtRON VRE vanA/vanB real-time PCR (iNtRON; iNtRON Biotechnology, Korea) assay, a multiplex real-time PCR method, was introduced. In this prospective study, we compared the iNtRON assay with the Seeplex VRE ACE detection kit (Seeplex; Seegene, Korea), a conventional multiplex PCR assay.
Methods
A chromogenic agar-based culture, in which pre-selected vancomycin-resistant enterococci (VRE) was grown and subsequently plated on blood agar with vancomycin disks, was regarded as the reference method. A total of 304 consecutive rectal swab specimens were tested for VRE by culture and by iNtRON and Seeplex PCR assays. For the PCR assays, specimens were enriched for 16-24 hr before PCR.
Results
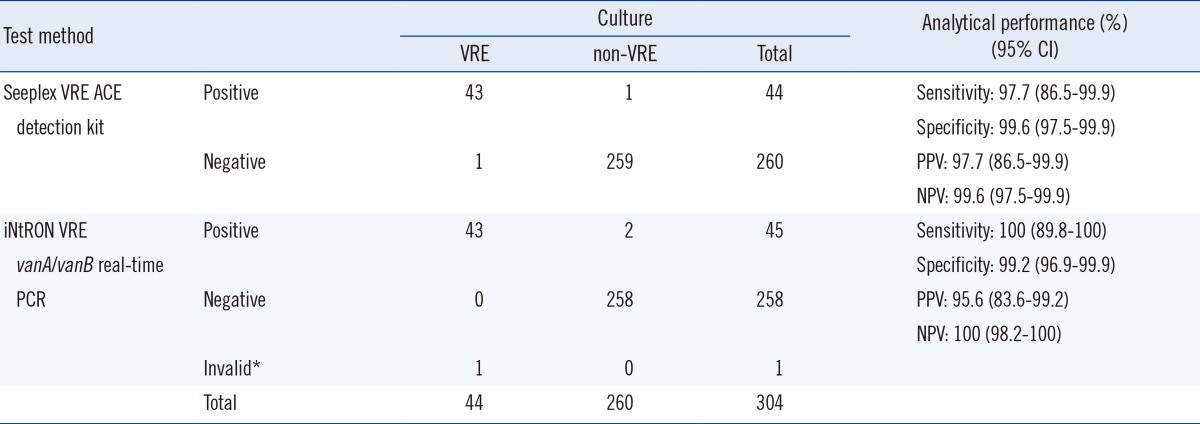
VRE were isolated from 44 (14.5%) specimens by chromogenic agar-based culture. The clinical sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the iNtRON assay were 100% (95% confidence interval: 89.8%-100%), 99.2% (96.9%-99.9%), 95.6% (83.6%-99.2%), and 100% (98.2%-100%), respectively, while those of the Seeplex assay were 97.7% (86.2%-99.9%), 99.6% (97.5%-99.9%), 97.7% (86.2%-99.9%), and 99.6% (97.5%-99.9%), respectively. The iNtRON assay had a detection limit of 3,159 copies/µL and 13,702 copies/µL for the vanA and vanB genes, respectively. No cross-reactivity was observed in 11 non-VRE bacterial culture isolates.
Go to : 
Vancomycin-resistant enterococci (VRE) have emerged as a significant cause of nosocomial infection. Vancomycin resistance in enterococcal species is conferred mainly by the vanA or vanB genes [1, 2]. Phenotypically, the vanA gene mediates a high-level of resistance to vancomycin and teicoplanin, whereas the vanB gene confers low- to moderate-level resistance to vancomycin only [3, 4, 5].
Infections with VRE have significant impacts on morbidity, mortality, length of hospital stay, and total costs [6, 7]. In addition, asymptomatic VRE colonization can provide a reservoir for dissemination and subsequent infection [8, 9]. Effective infection control and prevention can reduce the transmission of VRE. Therefore, rapid detection of VRE is important for the control and prevention of its nosocomial transmission.
Current techniques used for the detection of VRE include selective culture or the combination of culture with molecular detection of genes responsible for vancomycin resistance. Although culture is the reference method for confirmation of VRE, it is time-consuming and may delay isolation of patients carrying VRE [10, 11]. Molecular detection of resistance-related genes, on the other hand, requires a relatively short period of time.
The iNtRON VRE vanA/vanB real-time PCR assay (iNtRON; iNtRON Biotechnology, Seoul, Korea) was recently developed for rapid screening of VRE [12]. The iNtRON assay uses a real-time PCR format with TaqMan hydrolysis probes for the concurrent detection of vanA and vanB genes and an internal control. The present study aimed to evaluate the diagnostic accuracy of the iNtRON assay relative to the Seeplex VRE ACE detection kit (Seeplex; Seegene, Seoul, Korea), a multiplex end-point PCR assay, and the chromogenic agar-based culture method.
Go to : 
This study was conducted at a tertiary-care hospital in Seoul, Korea, and was approved by the Institutional Review Board of Samsung Medical Center. The study was carried out in a routine diagnostic laboratory setting and used non-selective, consecutive clinical specimens from Korean patients. From December 2010 to February 2011, we examined a total of 304 rectal swab specimens, for which VRE rectal examination had been requested for the purpose of VRE surveillance. Specimens came from patients who were transferred from another hospital, patients who were deemed to be high risk, or patients who were admitted to intensive care units. Rectal swabbing was performed by using a sterile transport system composed of two cotton swabs within an agar gel transport medium (COPAN, Amies, Italy). Two rectal swab specimens were collected at the same time, and each swab sample was randomly allocated to microbiology and molecular genetics laboratories for VRE culture and PCR, respectively.
Rectal swabs were directly inoculated onto a chromogenic agar plate (ChromID VRE agar, bioMérieux, Marcy I'Etoile, France) containing 8 µg/mL vancomycin and incubated aerobically at 35℃. The agar plates were screened for growth of presumptive colonies after 24 hr and 48 hr of incubation. For colonies resembling enterococci, gram staining and the pyrrolidonyl arylamidase (PYR) test were performed. Then, gram-positive, PYR-positive cocci were screened for vancomycin resistance. Colonies were inoculated onto blood agar plates containing a 30-µg vancomycin disk. Following an incubation period of 24 hr, colonies that demonstrated vancomycin resistance were submitted for identification and antimicrobial susceptibility testing.
The species of presumptive isolates were identified by the automated VITEK-2 system (bioMérieux). We performed the methyl D-glucopyranoside (MGP) test in order to distinguish Enterococcus gallinarum or E. casseliflavus from E. faecium. Antimicrobial susceptibility and minimum inhibitory concentrations (MICs) of vancomycin and teicoplanin were determined with the same instrument. Phenotypic identification of VRE isolates by culture is based on a MIC of ≥32 µg/mL [13].
For enrichment of VRE prior to PCR, one cotton swab was inoculated into Enterococcosel broth (Komed, Seongnam, Korea) containing 6 µg/mL vancomycin and incubated for 16 hr -24 hr at 35℃, after which 50 µL of culture broth was boiled at 100℃ for 10 min and centrifuged at 12,281 g for 5 min for DNA extraction. Then, 3 µL of supernatant was added to 17 µL of PCR mastermix, which consisted of 4 µL 5× VRE primer, 3 µL 8-methoxypsoralen, and 10 µL 2× Multiplex Master Mix (Seegene), for a final volume of 20 µL. PCR amplification was performed by using the following conditions: initial denaturation at 94℃ for 15 min; 35 cycles of denaturation at 94℃ for 30 sec, annealing at 60℃ for 1 min, extension at 72℃ for 1 min; and final extension at 72℃ for 10 min. vanA- and vanB- positive enterococci were included as external positive controls. Amplification products were detected by using capillary electrophoresis technology (Lab901 Screen Tape System; Lab901 Ltd, Loanhead, UK).
After enrichment of VRE in Enterococcosel broth containing 6 µg/mL vancomycin, 500 µL of the broth was centrifuged at 12,281 g for 5 min and the pellet was washed with distilled water. The pellet was incubated at 65℃ for 15 min with 50 µL sample preparation solution and then boiled at 95℃ for 10 min, followed by centrifugation at 12,281 g for 1 min. Five µL of the supernatant was then added to 10 µL of the 2× PCR mixture and 5 µL of the primer probe mixture. After vigorous vortexing and centrifugation, PCR was performed with the SLAN real-time PCR detection system (LG Lifescience, Seoul, Korea) using the following conditions: initial denaturation at 95℃ for 10 min, followed by 45 cycles of 15 sec at 95℃ and 30 sec at 55℃. The real-time PCR procedures included vanA- and vanB- positive and negative controls as well as an internal control in each run. In order to be counted as positive, amplification curves had to exhibit the typical sigmoid form and values between cycles 19 and 33. Invalid results showing no amplification in the internal control were retested by PCR with 10-fold dilutions.
The chromogenic agar-based culture method was regarded as the reference method for VRE screening. Enterococcus species that were demonstrated to be vancomycin resistant by bacterial identification and antimicrobial susceptibility tests were designated as VRE.
Concentrations of genomic DNA extracted from vanA-positive E. faecium type strain CCUG 36804 (Culture Collection, University of Göteborg, Sweden) and vanB-positive E. faecalis type strain CCARM 5025 (Culture Collection of Antibiotic-resistant Microbes, Korea) were measured by using a spectrophotometer (NanoDrop ND-1000, Thermo Fisher Scientific, Wilmington, DE, USA) and this was used for determining analytical sensitivity. Serial dilutions of the prepared genomic DNA were made from 102 to 10-9 ng/µL to determine the analytical sensitivity of the assay. Three replicates of each dilution step were performed. The lower detection limit was defined as the lowest concentration detected by the assay among three replicates.
The cross-reactivity of the iNtRON assay was assessed by using 11 different bacteria. E. gallinarum, E. casseliflavus, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Serratia marcesens, Proteus mirabilis, Salmonella species, Shigella species, Staphylococcus aureus, and S. epidermidis were obtained from ATCC (Manassas, VA, USA). The DNA of supplied samples was extracted and assayed with the iNtRON assay adhering to the same procedures used for sample processing.
Statistical analyses were performed by using the SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA) and the MedCalc statistical software, version 11.6 (Mariakerke, Belgium). We used inter-rater agreement statistics (Kappa calculation) to compare the detection of the vanA and vanB genes between the Seeplex and iNtRON assays. P values less than 0.05 were considered statistically significant. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence interval (CI) of PCR assays were evaluated in comparison with the results of the chromogenic agar-based cultures.
Go to : 
A total of 44 VRE were isolated from 304 samples, and the overall culture positivity rate was 14.5%. All of the VRE recovered were resistant to vancomycin (MIC≥32 µg/mL) and all isolates were E. faecium.
A comparison of the results of the iNtRON assay, Seeplex assay, and chromogenic agar-based culture is presented in Table 1. A total of 45 (14.8%) and 44 (14.5%) specimens were positive for vanA in the iNtRON and Seeplex assays, respectively. All specimens were negative for the vanB gene. One invalid result was observed by the iNtRON assay, which was vanA-positive VRE by Seeplex assay and chromogenic culture. The invalid result was excluded in the performance analysis. The positive-percent agreement of the iNtRON assay compared to the Seeplex assay was 100% (95% CI, 89.8 to 100), and the negative-percent agreement was 99.2% (95% CI, 96.9 to 99.9). The kappa value for the two methods was 0.97 (95% CI, 0.94 to 1.00; P<0.001).
Three specimens had discrepant results between the culture and PCR assays. Among them, two samples tested culture-negative, but vanA PCR-positive; one of those tested PCR-positive in both the iNtRON and the Seeplex assays, while the other tested positive only in the iNtRON assay. One sample tested positive by culture and in the iNtRON assay, but tested negative in the Seeplex assay.
Compared to the culture method, the iNtRON assay had an overall sensitivity of 100% and a specificity of 99.2%. The PPV and NPV were 95.6% and 100%, respectively. The Seeplex assay had an overall sensitivity of 97.7% and a specificity of 99.6%. The PPV and NPV were 97.7% and 99.6%, respectively (Table 1).
In tests for the detection limit of the iNtRON assay, the measured limit for vanA and vanB genes were 0.01 ng/µL (equal to 3,159 copies/µL) and 0.05 ng/µL (equal to 13,702 copies/µL), respectively. To evaluate the cross-reactivity and detection specificity, 11 different bacterial reference strains were tested. All assay results were negative and no non-specific positive reactions were observed.
Go to : 
The iNtRON assay was recently described as a rapid and useful method to monitor VRE-colonized or infected patients by Bae et al. [12]. They compared direct PCR and culture-based methods using stool specimens in patients undergoing follow-up VRE surveillance. Unique to our study, the iNtRON assay with enrichment broth was used as the initial screen for the vanA and vanB resistance determinants from rectal swab specimens. The present study demonstrated that the performance of multiplex real-time PCR following growth in enrichment broth is comparable to that of conventional end-point PCR and of chromogenic culture when screening for VRE using rectal swab specimens. In this study, the iNtRON assay showed perfect sensitivity and NPV (both 100%), while the specificity and PPV for vanA-type VRE (99.2% and 95.6%, respectively) were comparable to those of the Seeplex assay (99.6% and 97.7%, respectively). Previous studies that evaluated the performance of PCR for VRE screening have also shown good results for detection of the vanA gene [1, 11, 12, 14, 15, 16, 17].
Only two discordant culture-negative, but iNtRON assay PCR-positive results were observed among the vanA-positive specimens. Nonviable or viable but non-culturable (VBNC) Enterococcus spp. may cause culture-negative, but PCR-positive results [18, 19]. Enterococcal VBNC cells are a potential risk for human health in that they might constitute a reservoir of infectious bacteria involved in disease transmission and persistence. In addition, the difference in stool density of VRE in rectal swab samples can influence the sensitivity of the tests [11, 20]. A transport system for rectal swab samples consists of two cotton swabs; therefore, the VRE stool density in each swab may be different. The use of a cotton swab with a low density of VRE in culture or PCR assay may cause a false-negative test result. On the other hand, non-enterococcal isolates harboring vanA genes may contribute to false-positive PCR results. Albeit in limited numbers, Bacillus circulans, Arcanobacterium haemolyticum, Oerskovia turbata, and Staphylococcus aureus have been reported to acquire vanA genes [11, 21, 22, 23, 24, 25]. However, compared to the previous study on performance of the iNtRON assay [12], the present study did not detect significantly more patients as VRE carriers via this assay than culture methods alone. This may be due to differences among the surveillance specimens used for the study. We did not include the specimens that were obtained from patients for follow-up VRE surveillance. The iNtRON assay has an advantage in workload compared to the Seeplex assay. Given that the Seeplex assay requires agarosegel detection following PCR, the iNtRON assay requires less time and labor than does the Seeplex assay. The turnaround times of the iNtRON and Seeplex assays from DNA isolation to test results were less than 2.5 hr and 3.5 hr, respectively. In addition, iNtRON is a closed PCR system with a reduced chance of amplicon contamination.
The present study has some limitations. First, no vanB-type VRE were detected during the study period. In our study, pre-selected VRE strains on chromogen agar plates were subsequently cultured on blood agar plates with vancomycin disks. This would initially suppress vanB-type VRE with low to intermediate levels of vancomycin resistance. However, the prevalence of vanB-type VRE in Korea is extremely low; the outbreak or colonization of vanB-type VRE has been reported only in a limited number of studies [26, 27, 28, 29]. Second, the present study employed only multiplex real-time PCR assays following culture in enrichment broth. We did not undertake direct detection of vanA/vanB genes in rectal swabs without enrichment.
To conclude, the overall performance of the iNtRON assay is comparable to that of the Seeplex assay and a chromogenic agar-based culture method for prompt identification of VRE-colonized patients in hospitals. This assay could be an alternative or supportive method for effective control of nosocomial VRE infection.
Go to : 
Acknowledgements
This study was supported by LG Lifescience, Korea. The sponsor had no involvement in the study design, data interpretation, or writing of the manuscript.
Go to : 
References
1. Babady NE, Gilhuley K, Cianciminio-Bordelon D, Tang YW. Performance characteristics of the Cepheid Xpert vanA assay for rapid identification of patients at high risk for carriage of vancomycin-resistant Enterococci. J Clin Microbiol. 2012; 50:3659–3663. PMID: 22972822.
2. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006; 42(S1):S25–S34. PMID: 16323116.

3. Bell JM, Paton JC, Turnidge J. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J Clin Microbiol. 1998; 36:2187–2190. PMID: 9665988.

4. Mak A, Miller MA, Chong G, Monczak Y. Comparison of PCR and culture for screening of vancomycin-resistant Enterococci: highly disparate results for vanA and vanB. J Clin Microbiol. 2009; 47:4136–4137. PMID: 19846635.
5. Park IJ, Lee WG, Lee H, Yong D, Lee K, Kim EC, et al. Mechanism of vanB phenotype in vancomycin-resistant Enterococci carrying vanA gene. Korean J Lab Med. 2006; 26:412–417. PMID: 18156760.

6. DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005; 41:327–333. PMID: 16007529.

7. Salgado CD, Farr BM. Outcomes associated with vancomycin-resistant enterococci: a meta-analysis. Infect Control Hosp Epidemiol. 2003; 24:690–698. PMID: 14510253.

8. Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, et al. The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998; 158:1127–1132. PMID: 9605785.
9. Perl TM. The threat of vancomycin resistance. Am J Med. 1999; 106:26S–37S. discussion 48S-52S. PMID: 10348061.

10. Roger M, Faucher MC, Forest P, St-Antoine P, Coutlée F. Evaluation of a vanA-specific PCR assay for detection of vancomycin-resistant Enterococcus faecium during a hospital outbreak. J Clin Microbiol. 1999; 37:3348–3349. PMID: 10488203.
11. Seo JY, Kim PW, Lee JH, Song JH, Peck KR, Chung DR, et al. Evaluation of PCR-based screening for vancomycin-resistant enterococci compared with a chromogenic agar-based culture method. J Med Microbiol. 2011; 60:945–949. PMID: 21459908.

12. Bae MH, Kim J, Sung H, Jeong YS, Kim MN. Evaluation of iNtRON VRE vanA/vanB real-time PCR for follow-up surveillance of VRE-infected or colonized patients. Diagn Microbiol Infect Dis. 2013; 77:292–295. PMID: 24094836.
13. Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013; 1:CD009593. PMID: 23440842.

14. Cekin Y, Erman Daloğlu A, Oğünç D, Ozhak Baysan B, Dağlar D, Inan D, et al. Evaluation of vancomycin resistance 3 multiplexed PCR assay for detection of vancomycin-resistant enterococci from rectal swabs. Ann Lab Med. 2013; 33:326–330. PMID: 24003422.

15. Kim TS, Kwon HL, Song SH, Song KH, Kim HB, Park KU, et al. Real-time PCR surveillance of vanA for vancomycin-resistant Enterococcus faecium. Mol Med Rep. 2012; 6:488–492. PMID: 22710424.

16. Werner G, Serr A, Schütt S, Schneider C, Klare I, Witte W, et al. Comparison of direct cultivation on a selective solid medium, polymerase chain reaction from an enrichment broth, and the BD GeneOhm™ VanR Assay for identification of vancomycin-resistant enterococci in screening specimens. Diagn Microbiol Infect Dis. 2011; 70:512–521. PMID: 21767707.

17. Stamper PD, Cai M, Lema C, Eskey K, Carroll KC. Comparison of the BD GeneOhm VanR assay to culture for identification of vancomycin-resistant enterococci in rectal and stool specimens. J Clin Microbiol. 2007; 45:3360–3365. PMID: 17704282.

18. Lleò MM, Bonato B, Tafi MC, Signoretto C, Boaretti M, Canepari P. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol. 2001; 91:1095–1102. PMID: 11851818.

19. Lleò MM, Bonato B, Signoretto C, Canepari P. Vancomycin resistance is maintained in enterococci in the viable but nonculturable state and after division is resumed. Antimicrob Agents Chemother. 2003; 47:1154–1156. PMID: 12604561.

20. D'Agata EM, Gautam S, Green WK, Tang YW. High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin Infect Dis. 2002; 34:167–172. PMID: 11740703.
21. Patel R. Enterococcal-type glycopeptide resistance genes in non-enterococcal organisms. FEMS Microbiol Lett. 2000; 185:1–7. PMID: 10731599.

22. Fontana R, Ligozzi M, Pedrotti C, Padovani EM, Cornaglia G. Vancomycin-resistant Bacillus circulans carrying the vanA gene responsible for vancomycin resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1997; 16:473–474. PMID: 9248754.
23. Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin Infect Dis. 2008; 46:668–674. PMID: 18257700.

24. Power EG, Abdulla YH, Talsania HG, Spice W, Aathithan S, French GL. vanA genes in vancomycin-resistant clinical isolates of Oerskovia turbata and Arcanobacterium (Corynebacterium) haemolyticum. J Antimicrob Chemother. 1995; 36:595–606. PMID: 8591934.
25. Ligozzi M, Lo Cascio G, Fontana R. vanA gene cluster in a vancomycin-resistant clinical isolate of Bacillus circulans. Antimicrob Agents Chemother. 1998; 42:2055–2059. PMID: 9687406.
26. Lee WG, Jernigan JA, Rasheed JK, Anderson GJ, Tenover FC. Possible horizontal transfer of the vanB2 gene among genetically diverse strains of vancomycin-resistant Enterococcus faecium in a Korean hospital. J Clin Microbiol. 2001; 39:1165–1168. PMID: 11230450.
27. Ko KS, Baek JY, Lee JY, Oh WS, Peck KR, Lee N, et al. Molecular characterization of vancomycin-resistant Enterococcus faecium isolates from Korea. J Clin Microbiol. 2005; 43:2303–2306. PMID: 15872259.
28. Won D, Hong KH, Yun K, Sung H, Kim MN. Occurrence of a PCR-positive but culture-negative case forvanB vancomycin-resistant Enterococci in stool surveillance. Lab Med Online. 2013; 3:264–268.
29. Seol CA, Park JS, Sung H, Kim MN. Co-colonization of vanA and vanB Enterococcus faecium of clonal complex 17 in a patient with bacteremia due to vanA E. faecium. Diagn Microbiol Infect Dis. 2014; 79:141–143. PMID: 24674093.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download