Abstract
Background
Methods
Results
Acknowledgments
References
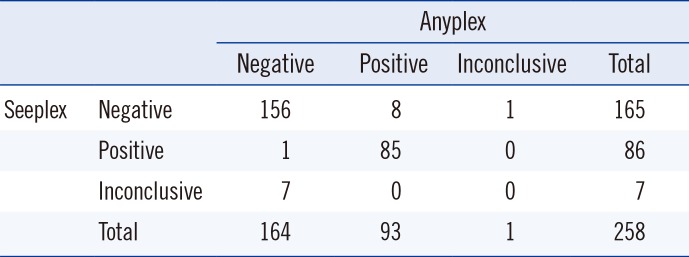
Table 1
Comparison of BRAF V600E mutation-detection results for the Seeplex and Anyplex assays
| Anyplex | |||||
|---|---|---|---|---|---|
| Negative | Positive | Inconclusive | Total | ||
| Seeplex | Negative | 156 | 8 | 1 | 165 |
| Positive | 1 | 85 | 0 | 86 | |
| Inconclusive | 7 | 0 | 0 | 7 | |
| Total | 164 | 93 | 1 | 258 | |
Table 2
Comparison of BRAF V600E mutation detection using the Seeplex and Anyplex assays, with cytological diagnoses (n=258) and post-thyroidectomy histological diagnoses (n=91)*
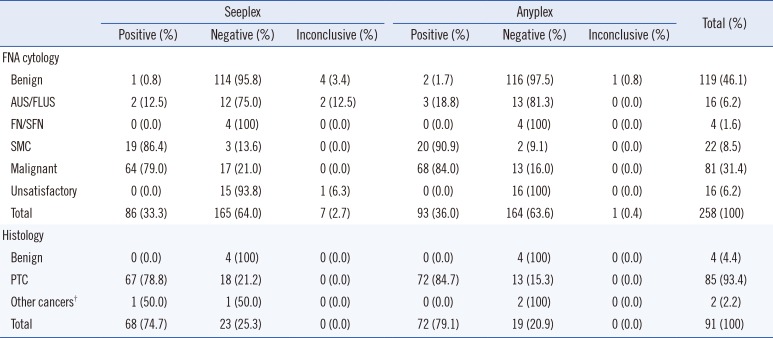
*Two specimens contained the BRAF V600E mutation as detected by mutant enrichment with 3'-modified oligonucleotide (MEMO) sequencing; however the mutation was not detected by dual-priming oligonucleotide (DPO) PCR or DPO real-time PCR, and these were diagnosed as benign on FNA cytology. Histopathological evaluations with surgical thyroidectomy for these cases were not performed; †Other cancers included one minimally-invasive follicular carcinoma and one medullary thyroid carcinoma.
Abbreviations: FNA, fine needle aspirates; AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; FN/SFN, follicular or oncocytic (Hürthle cell) neoplasm/suspicious for follicular or oncocytic (Hürthle cell) neoplasm; SMC, suspicious for malignant cells; PTC, papillary thyroid cancer; NT, not tested.
Table 3
Conflicting results between Seeplex and Anyplex assays (n=9) were resolved using MEMO sequencing, FNA cytology, and post-thyroidectomy histological analyses
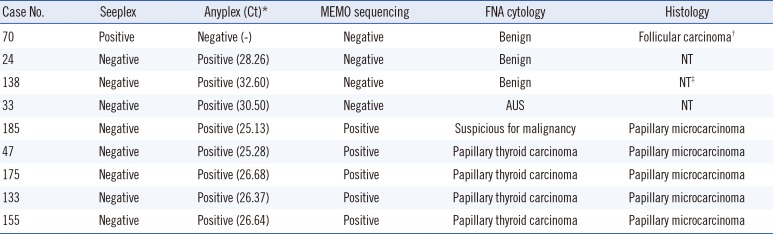
*Ct values <33 were considered positive; †Minimally-invasive follicular carcinoma; ‡Post-thyroidectomy histological findings were unavailable; however, follow-up FNA cytology results after 18 months indicated this case was benign.
Abbreviations: Ct, cycle threshold; MEMO, mutant enrichment with 3'-modified oligonucleotide; FNA, fine needle aspirates; NT, not tested; AUS, atypia of undetermined significance.
Table 4
The limits of detection for the Anyplex assay
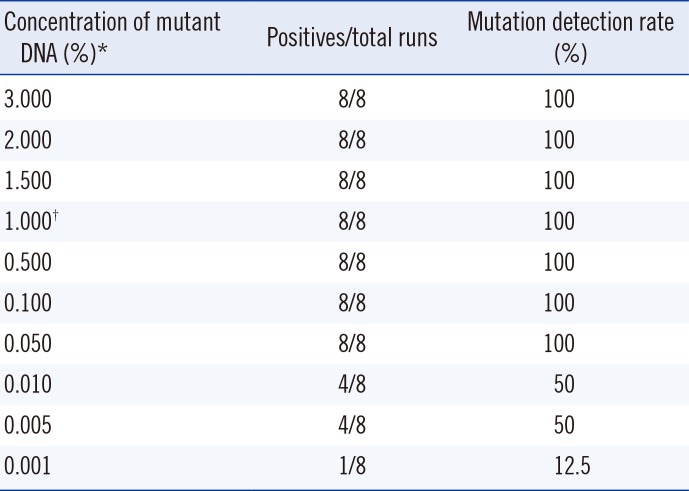
*The SNU-790 BRAF V600E-positive cell line was serially diluted with the DMPK-M BRAF V600E-negative cell line; †The limit of detection claimed by the manufacturer was 1.000% of BRAF mutations. All results were positive when the concentration was 0.050%. The limit of detection as determined by probit analysis was 0.046% (95% confidence interval:0.019-0.532). Reproducibility was verified at 1.000% and lower concentrations.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download