This article has been
cited by other articles in ScienceCentral.
The Rh blood group system is the second most clinically significant blood group system after the ABO blood group system [
1]. Extremely weak D variants known as DEL, which are weaker than Du, are hardly detectable by basic serologic typing except absorption-elution techniques and frequently require molecular studies for confirmation [
2]. According to the previous review, the 678 DELs found by elution or DNA detection were genotyped, and more than 98% (667/678) presented the
RHD (c.1227G>A) allele, which predominates in East Asians [
3]. Thus, it is known as the "Asian type". Here, we present a case of primary anti-D alloimmunization induced by the transfusion of packed red blood cells (RBCs) from two Korean donors, both of whom coincidentally had DEL phenotypes.
A 64-yr-old Caucasian male was admitted to our hospital for surgery for prostate adenocarcinoma. His blood type was group A, D-negative. The patient had no history of transfusion. Two days after admission, two units of crossmatch-compatible blood group A, D-negative packed RBCs from two separate donors were administered. The result of a pre-transfusion antibody screening test (BioVue, Ortho Clinical Diagnostics, Raritan, NJ, USA), an indirect antiglobulin test with column agglutination, was negative. No initial adverse effect of transfusion was observed. At day 12, the antibody screening test became positive and anti-D was identified in the recipient's serum (Resolve Panel A, Ortho Clinical Diagnostics). An autocontrol and a direct antiglobulin test (DAT) showed no visible agglutination.
Anti-D development after transfusion in this patient was unexplainable, and possible analytical errors were ruled out. An antibody screening test performed by using previously taken blood samples yielded strongly positive results (4+) on days 7, 9, and 12 and a negative result on day 5. This implied that anti-D developed between day 5 and day 7.
The remaining pre-sealed portions of two transfused RBC units were sent to the Korean Rare Blood Program Reference Laboratory (Seoul National University Bundang Hospital) for confirmation of the
RHD variants. The
RHD genotype was analyzed according to the previously described methods [
4]. Surprisingly, the RhD genotype results for both donors were
RHD (c.1227G>A). They presented the Ccee and CCEe phenotypes (
Table 1). The two
RHD (c.1227G>A)-positive RBC units were transfused at day 2 after admission, and the above observations suggest that anti-D alloimmunization caused anti-D to be detected more than 3 days later.
Table 1
Blood group immunogenetic results of two donors
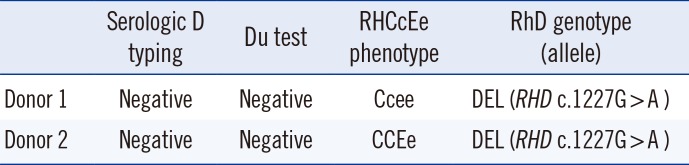
|
Serologic D typing |
Du test |
RHCcEe phenotype |
RhD genotype (allele) |
|
Donor 1 |
Negative |
Negative |
Ccee |
DEL (RHD c.1227G > A ) |
|
Donor 2 |
Negative |
Negative |
CCEe |
DEL (RHD c.1227G > A ) |

DEL can cause anti-D alloimmunization despite small amounts of D antigens on RBCs. Several cases of anti-D alloimmunization caused by transfusion from DEL donors have been reported [
456] (
Table 2). Although 16% of serologically D-negative Korean blood donors were known to be DEL, only one patient of anti-D alloimmunization has been reported in Korea [
4]. In our case, the patient received serologically D-negative RBCs from two donors, who were later shown to have the DEL phenotype by
RHD genotyping. Two separate serologically D-negative RBC units with a DEL phenotype may not be a coincidence. In Germany and Upper Austria,
RHD genotyping of D-negative donors is routinely performed as a screening method at first-time donation [
78]. The prevalence of
RHD gene carriers was 0.21% for six years, and approximately a half of them had a DEL phenotype, according to the data from Germany [
7]. In Upper Austria, of 23,330 serologically D-negative samples, 94 showed one or more
RHD markers from among 20
RHD markers located in exons 4, 7, and 10 [
8]. However, according to the national transfusion guidelines from South Korea, serologically D-negative units are not tested for true D-negative and DEL phenotypes. Therefore, it is possible for DEL-type packed RBC units to be transfused to D-negative recipients. If
RHD PCR is adopted as an initial screening method for D-negative blood, the number of cases with DEL phenotypes being serologically mistyped as general D-negative will decrease. However, it would increase costs and require additional time.
Table 2
Characteristics of anti-D immunization by the DEL RBCs in literature
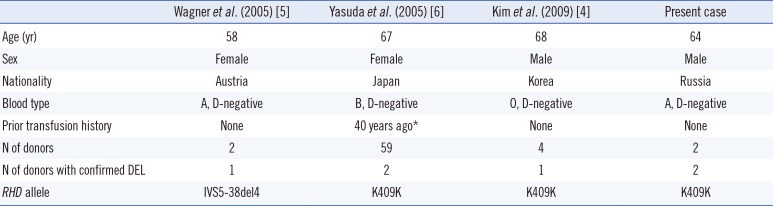
|
Wagner et al. (2005) [5] |
Yasuda et al. (2005) [6] |
Kim et al. (2009) [4] |
Present case |
|
Age (yr) |
58 |
67 |
68 |
64 |
|
Sex |
Female |
Female |
Male |
Male |
|
Nationality |
Austria |
Japan |
Korea |
Russia |
|
Blood type |
A, D-negative |
B, D-negative |
O, D-negative |
A, D-negative |
|
Prior transfusion history |
None |
40 years ago*
|
None |
None |
|
N of donors |
2 |
59 |
4 |
2 |
|
N of donors with confirmed DEL |
1 |
2 |
1 |
2 |
|
RHD allele |
IVS5-38del4 |
K409K |
K409K |
K409K |

Previous investigations reported that the presence of
RHD genes was strongly related to RhC phenotype, with a high frequency of RhC(+) in serologically RhD-negative blood [
910]. In an earlier study of D-negative Koreans, all except one (97.6%) of 42 "Asian type (c.1227G>A)" individuals showed a RhC phenotype [
9]. Our study also showed the RhC(+) phenotype in both donors, who have Ccee and CCEe phenotypes. Thus, we agree with Wang et al. [
10] that a laboratory protocol for blood banks that includes RhC phenotyping and confirmatory
RHD PCR would be helpful for detecting DEL RBCs. Additional nationwide Korean data concerning the incidence of the RhC(+) phenotype in DEL individuals needs to be collected.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download