Abstract
We compared the activities of tedizolid to those of linezolid and other commonly used antimicrobial agents against gram-positive cocci recovered from patients with skin and skin structure infections (SSSIs) and hospital-acquired pneumonia (HAP) in Korean hospitals. Gram-positive isolates were collected from 356 patients with SSSIs and 144 patients with HAP at eight hospitals in Korea from 2011 to 2014. SSSIs included impetigo, cellulitis, erysipelas, furuncles, abscesses, and infected burns. Antimicrobial susceptibility was tested by using the CLSI agar dilution method. All of the gram-positive isolates were inhibited by ≤1 µg/mL tedizolid. The minimum inhibitory concentration [MIC]90 of tedizolid was 0.5 µg/mL for methicillin-resistant Staphylococcus aureus, which was 4-fold lower than that of linezolid. Tedizolid may become a useful option for the treatment of SSSIs and HAP caused by gram-positive bacteria.
Skin and skin structure infections (SSSIs) are common problems in both inpatients and outpatients. The vast majority of SSSIs are caused by gram-positive organisms that are normal flora on the skin of human beings. Staphylococci and streptococci cause majority of gram-positive infections [1]. A recent increase in staphylococcal infections caused by methicillin-resistant Staphylococcus aureus (MRSA) has resulted in a significant increase of cases of MRSA pneumonia in the health care setting, especially in the chronically ill population [2]. Vancomycin has been the cornerstone of treatment for MRSA infections. However, recently, vancomycin-resistant S. aureus and linezolid-resistant Staphylococcus strains have emerged [345]. These strains pose significant challenges to the clinical treatment of infections caused by these organisms. Tedizolid offers broad in vitro activity against gram-positive pathogens, including MRSA and strains resistant to vancomycin or linezolid, and has greater potency than other drugs of its class [67]. It was specifically designed to be active against linezolid-resistant S. aureus, including strains containing the multidrug-resistant cfr gene [8].
Tedizolid phosphate was recently approved by the U.S. Food and Drug Administration to treat patients with acute bacterial SSSI caused by S. aureus, various Streptococcus species, and Enterococcus. In addition, planned studies will investigate the potential role of tedizolid in the treatment of community-acquired bacterial pneumonia and hospital-acquired pneumonia (HAP) [9]. We published a previous report focusing on the activity of tedizolid against collections of clinical isolates in a single institution, but it was not characterized by infection type [10]. Therefore, the present study aimed to compare the activities of tedizolid to those of linezolid and other commonly used antimicrobial agents against gram-positive cocci recovered from patients with SSSIs and HAP in Korean hospitals.
Non-duplicated aerobic and anaerobic gram-positive isolates were collected from clinical specimens of 356 patients with SSSIs and 144 patients with HAP at eight hospitals in Seoul and Gyeonggi province, Korea from 2011 to 2014. SSSIs included impetigo, cellulitis, erysipelas, furuncles, abscesses, and infected burns [111]. HAP was defined as pneumonia that occurred 48 hr or more after admission.
Species were identified by using conventional methods or the Vitek 2 system (bioMérieux, Marcy l'Etolile, France). Antimicrobial susceptibility was tested by using the CLSI agar dilution method [1213]. Mueller-Hinton agar was used as a growth medium (Becton Dickinson, Cockeysville, MD, USA) for testing Staphylococcus spp. and Enterococcus spp.: Mueller-Hinton agar supplemented with 5% sheep blood for Streptococcus spp.; Brucella agar (Becton Dickinson) supplemented with 5 µg/mL hemin, 1 µg/mL vitamin K1; and 5% laked sheep blood for anaerobic bacteria. Tedizolid and linezolid (Dong-A ST, Seoul, Korea); erythromycin, tetracycline, oxacillin and penicillin G (Sigma Chemical, St. Louis, MO, USA); piperacillin and tazobactam (Yuhan, Seoul, Korea); clindamycin (Korea Upjohn, Seoul, Korea); levofloxacin (Daiichi Pharmaceutical, Tokyo, Japan); cefotetan (Daiichi Pharmaceutical); ampicillin and gentamicin (Chong Kun Dang, Seoul, Korea); cefoxitin and imipenem (Merck Sharp & Dohme, Rahway, NJ, USA); meropenem (Sumitomo, Tokyo, Japan); metronidazole (ChoongWae, Seoul, Korea); trimethoprim and sulfamethoxazole (Dong Wha, Seoul, Korea); vancomycin (Daewoong, Seoul, Korea); and teicoplanin (Sanofi Aventis, Bridgewater, NJ, USA) were used as antimicrobial powders. American type culture collection strains of S. aureus (ATCC29213), E. faecalis (ATCC 29212), S. pneumoniae (ATCC 49619), Bacteroides fragilis (ATCC 25285), and B. thetaiotaomicron (ATCC 29741) were used as reference strains. The non-meningeal breakpoints of penicillin G and cefotaxime were used for S. pneumoniae. This study used the breakpoints of tedizolid suggested by the US Food and Drug Administration [14].
All of the aerobic and anaerobic gram-positive isolates in patients with SSSIs were inhibited by ≤1 µg/mL tedizolid (Table 1). The most potent drugs against MRSA were tedizolid (minimum inhibitory concentration [MIC]90=0.5 µg/mL), linezolid (MIC90=2 µg/mL), and vancomycin (MIC90=2 µg/mL). The MIC range of tedizolid was 0.125 to 0.5 µg/mL for MRSA, while that of linezolid was 0.25 to 4 µg/mL. The MIC90s of tedizolid were 0.5 µg/mL for both MRSA and methicillin-susceptible S. aureus (MSSA) and ≤0.125 µg/mL for coagulase-negative staphylococci, which were 2- to 4-fold lower than those of linezolid. These MIC values were similar to those described in previous reports [1015]. The MICs of tedizolid were 0.25 µg/mL for all three vancomycin-intermediate S. aureus isolates.
The MIC ranges of tedizolid were 0.25 to 0.5 µg/mL for Enterococcus, while those of linezolid were 0.5 to 2 µg/mL. Tedizolid inhibited all vancomycin-resistant Enterococcus at 0.5 µg/mL. When the meningeal breakpoint was applied, most of the pneumococcal isolates tested were not susceptible to penicillin G or cefotaxime. However, the MIC range of tedizolid was 0.25 to 1 µg/mL, and the MIC90 (0.5 µg/mL) was 4-fold lower than that of linezolid. Tedizolid inhibited all the isolates of viridans Streptococcus spp. and β-hemolytic streptococci such as S. pyogenes and S. agalactiae at 0.5 µg/mL.
Tedizolid had excellent activity against gram-positive anaerobes recovered from SSSIs (Table 1). The MIC ranges of tedizolid were 0.06 to 1 µg/mL for Finegoldia magna and ≤0.06 to 0.25 µg/mL for the other Peptostreptococcus spp. The MIC90 values for these organisms were 0.5 and 0.25 µg/mL, respectively, which were 4-8 fold lower than those of linezolid. All the Clostridium spp. isolates were inhibited by tedizolid at 0.5 µg/mL.
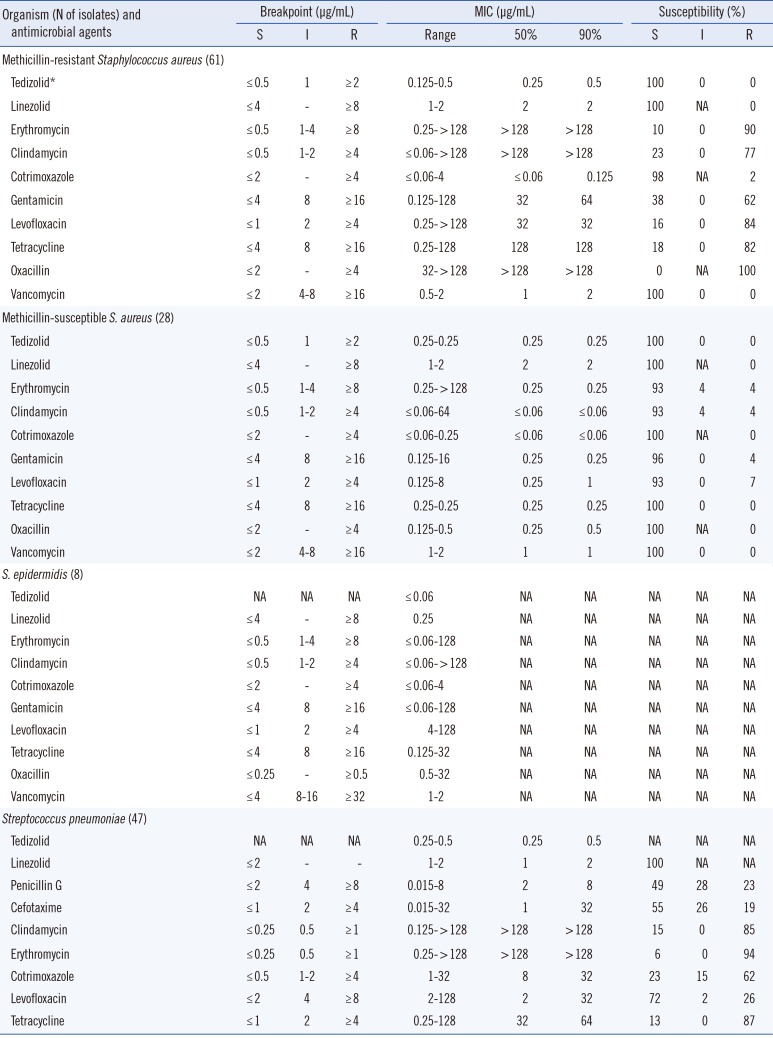
All the gram-positive isolates in patients with HAP were inhibited by ≤0.5 µg/mL tedizolid (Table 2). The MIC ranges of tedizolid were 0.125 to 0.5 µg/mL for MRSA and 0.25 µg/mL for MSSA. The MIC90 values of tedizolid were 0.25, 0.5, and 0.5 µg/mL for MSSA, MRSA, and pneumococci, respectively, which were 4- to 8-fold lower than those of linezolid.
In summary, the MIC values of tedizolid in this study were not significantly different according to type of infection. All organisms tested were susceptible to tedizolid, nevertheless the breakpoint of tedizolid is 4- or 8-fold lower than that of linezolid. Tedizolid is a potent agent with high in vitro activity against common aerobic and anaerobic gram-positive pathogens in SSSIs and HAP. Tedizolid may become a useful option for the treatment of SSSIs and HAP.
References
1. Rajan S. Skin and soft-tissue infections: classifying and treating a spectrum. Cleve Clin J Med. 2012; 79:57–66. PMID: 22219235.

2. Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008; 46(S5):S378–S385. PMID: 18462093.
3. Mendes RE, Flamm RK, Hogan PA, Ross JE, Jones RN. Summary of linezolid activity and resistance mechanisms detected during the 2012 LEADER surveillance program for the United States. Antimicrob Agents Chemother. 2014; 58:1243–1247. PMID: 24323470.

4. Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother. 2013; 68:4–11. PMID: 22949625.
5. Moravvej Z, Estaji F, Askari E, Solhjou K, Naderi Nasab M, Saadat S. Update on the global number of vancomycin-resistant Staphylococcus aureus (VRSA) strains. Int J Antimicrob Agents. 2013; 42:370–371. PMID: 23880172.
6. Rodríguez-Avial I, Culebras E, Betriu C, Morales G, Pena I, Picazo JJ. In vitro activity of tedizolid (TR-700) against linezolid-resistant staphylococci. J Antimicrob Chemother. 2012; 67:167–169. PMID: 21954458.
7. Shaw KJ, Poppe S, Schaadt R, Brown-Driver V, Finn J, Pillar CM, et al. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob Agents Chemother. 2008; 52:4442–4447. PMID: 18838596.

8. Moellering RC Jr. Tedizolid: a novel oxazolidinone for Gram-positive infections. Clin Infect Dis. 2014; 58(S1):S1–S3. PMID: 24343826.

9. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health Syst Pharm. 2014; 71:621–633. PMID: 24688035.

10. Yum JH, Choi SH, Yong D, Chong Y, Im WB, Rhee DK, et al. Comparative in vitro activities of torezolid (DA-7157) against clinical isolates of aerobic and anaerobic bacteria in South Korea. Antimicrob Agents Chemother. 2010; 54:5381–5386. PMID: 20837761.
11. US Food and Drug Administration. Guidance for industry: acute bacterial skin and skin structure infections: developing drugs for treatment. US Food and Drug Administration;2010.
12. Goldstein EJ, Solomkin JS, Citron DM, Alder JD. Clinical efficacy and correlation of clinical outcomes with in vitro susceptibility for anaerobic bacteria in patients with complicated intra-abdominal infections treated with moxifloxacin. Clin Infect Dis. 2011; 53:1074–1080. PMID: 21998288.

13. Brook I, Wexler HM, Goldstein EJ. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013; 26:526–546. PMID: 23824372.

14. Sivextro (tedizolid) Prescribing information. Lexington, MA: Cubist Pharmaceuticals;2014.
15. Thomson KS, Goering RV. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant Staphylococcus aureus strains of diverse epidemiological origins. Antimicrob Agents Chemother. 2013; 57:2892–2895. PMID: 23571550.
Table 1
Comparative in vitro activities of tedizolid and other antimicrobial agents against bacteria recovered from patients with skin and skin structure infections
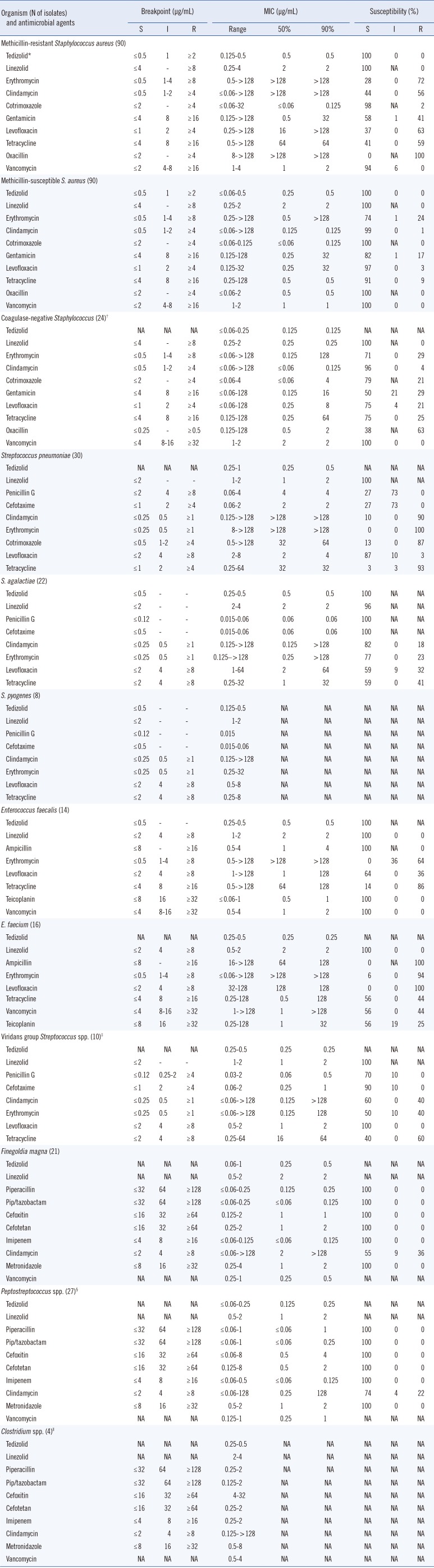
*FDA breakpoints were used for tedizolid; †Staphylococcus epidermidis (N=22), S. caprae (N=1), S. warneri (N=1); ‡Streptococcus mitis (N=6), S. anginosus (N=2), S. constellatus (N=2); §P. asaccharolyticus (N=11), P. micros (N=7), Anaerococcus prevotii (N=8), P. anaerobius (N=1); ∥C. perfringens (N=2), C. ramosum (N=2).
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; I, intermediate; R, resistant; NA, not available/applicable; Pip/tazobactam, piperacillin/tazobactam.
Table 2
Comparative in vitro activities of tedizolid and other antimicrobial agents against bacteria recovered from patients with hospital-acquired pneumonia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download