This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The Spectra Optia (SPO) is a novel continuous-flow centrifugal apheresis system based on the COBE Spectra (CSP) platform. There have been few attempts to validate the advantages of the SPO. We performed a retrospective study comparing the two cell separators for therapeutic plasma exchange (TPE) procedures in kidney transplant (KT) patients and seeing efficacy and safety.
Methods
We analyzed 720 TPE procedures performed between August 2012 and July 2014. Procedures included desensitization TPE before KT and TPE for the management of acute and chronic antibody-mediated graft rejection. Demographic characteristics, operational TPE variables, and laboratory data were analyzed.
Results
Demographic characteristics for the SPO (n=389) and CSP (n=331) groups did not differ significantly. The procedure time to exchange one plasma volume was 94.2±10.3 min in the SPO group and 100.4±11.2 min in the CSP group (P<0.001). The plasma removal efficiency (PRE) was 92.5±4.9% in the SPO group and 83.2±3.7% in the CSP group (P<0.001). There were no significant differences across the two apheresis systems for changes in hematologic parameters.
Conclusions
Compared with the CSP, the SPO was associated with an improved PRE and a shorter procedure time to exchange one plasma volume. Our results in KT patients show that the SPO is superior to the CSP in TPE procedures.
Go to :

Keywords: Apheresis, Plasma exchange, Kidney transplantation, Efficacy
INTRODUCTION
Despite previous limitations for solid organ transplantation due to ABO incompatibility, current medical practice has demonstrated increasingly successful outcomes using therapeutic plasmapheresis. In Japan, ABO-incompatible kidney transplantation (ABOiKT) has been performed in more than 1,000 patients since 1989, and in recent years, it has accounted for approximately 18% of all living donor kidney transplants (KTs) [
1]. As adjunct therapy, the routine use of rituximab, a monoclonal antibody directed against CD20 on B cells, has been shown to improve clinical outcomes following ABOiKT. The clinical literature has repeatedly shown that outcomes following ABOiKT are comparable to those following ABO-compatible KT [
2345]. In all the published desensitization protocols before ABOiKT, recipients underwent 3-6 sessions of therapeutic plasma exchange (TPE) to reduce anti-A or anti-B titers. ABOiKT is currently an American Society for Apheresis Category II indication for TPE [
6].
Plasma exchange is performed by using automated devices designed with specialized instruments for blood withdrawal, anticoagulation, separation, and blood return, as well as compartments for replacement fluid and separated substances. The Spectra Optia (SPO) is a new continuous-flow centrifugal apheresis system developed by Terumo BCT (Tokyo, Japan) in 2007 that is based on the COBE Spectra (CSP) platform. It first became available in Korea in 2012. The SPO incorporates an improved automated interface management system, a graphical user interface, better data management with processing options, and simplified, ready-to-use tubing sets. Since the SPO was first introduced, two comparative analyses of the efficacy of plasmapheresis using the CSP and SPO were published [
78]. The trials compared efficacy and safety in each patient, using each machine in a paired, non-blinded clinical trial design. These studies indicated the superiority of the SPO, and concluded that it was acceptable for regular use in clinical TPE. The studies were, however, limited by the small number of patients enrolled. In our study, we retrospectively compared TPE in KT patients using the SPO (n=389) and the CSP (n=331) on a larger scale in terms of efficacy and safety.
Go to :

METHODS
1. Patient selection
We performed a retrospective analysis to compare TPE procedures using the SPO and the CSP (Gambro BCT, Deerfield, IL, USA) at Severance Hospital (affiliated with Yonsei University Health System), Seoul, Korea. The TPE procedures were related to KT and included desensitization TPE for ABOiKT or lymphocyte crossmatch-positive KT and acute or chronic antibody-mediated graft rejection. The device was selected randomly for each procedure, not for each patient; thus, it was possible that patients underwent TPE with both devices, since each patient underwent multiple procedures. All procedures were performed between August 2012 and July 2014.
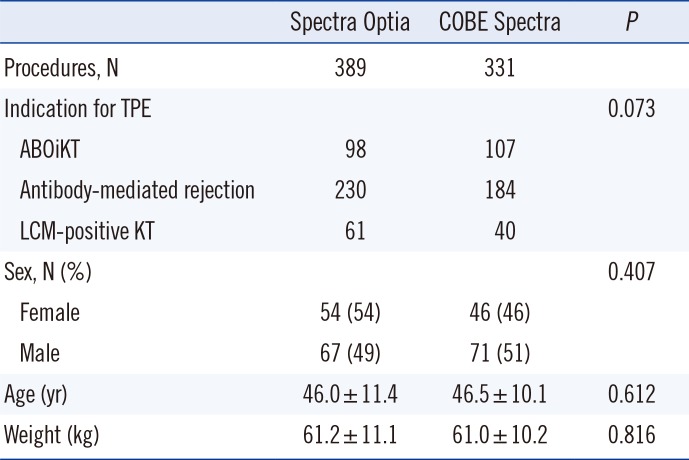
A total of 720 procedures were reviewed; 389 used the SPO, and 331 used the CSP. There were no significant differences in patient characteristics, including sex, age, and weight. Clinical diagnoses for TPE in the SPO group were ABOiKT (n=98), lymphocyte crossmatch-positive KT (n=61), and antibody-mediated rejection (n=230). In the CSP group, clinical diagnoses for TPE were ABOiKT (n=107), lymphocyte crossmatch-positive KT (n=40), and antibody-mediated rejection (n=184). There was no statistical differences in clinical diagnoses for TPE between the SPO and CSP groups (
Table 1).
Table 1
Procedures and patient characteristics
|
Spectra Optia |
COBE Spectra |
P
|
|
Procedures, N |
389 |
331 |
|
|
Indication for TPE |
|
|
0.073 |
|
ABOiKT |
98 |
107 |
|
Antibody-mediated rejection |
230 |
184 |
|
LCM-positive KT |
61 |
40 |
|
Sex, N (%) |
|
|
0.407 |
|
Female |
54 (54) |
46 (46) |
|
Male |
67 (49) |
71 (51) |
|
Age (yr) |
46.0 ± 11.4 |
46.5 ± 10.1 |
0.612 |
|
Weight (kg) |
61.2 ± 11.1 |
61.0 ± 10.2 |
0.816 |

2. Study design
In all TPE procedures, acid citrate dextrose-A (ACD-A) anticoagulation and dual vascular access were used, as previously described [
9]. The target volume of the procedure was 1.0 to 1.5 plasma volumes (PV), which theoretically leads to approximately 20-40% of the residual relative concentration [
10]. One PV was removed from each patient, and 100% replacement was administered using 5% albumin and AB-blood group fresh frozen plasma (FFP). The inlet:anticoagulant ratio (concentration of anticoagulant provided by the extracorporeal circuit) was 1:12 in the case of 5% albumin, and 1:15 in the case of FFP. All patients who underwent TPE were administered 1 ampule (10 mL) of 10% calcium gluconate (Daihan Pham Co. Ltd, Seoul, Korea) to prevent hypocalcemia, which is one of the most common complications of TPE [
111213]. After each procedure, the parameter shown in the result screen of the instrument, including whole blood flow rate, procedure time, PV processed, PV removed, used ACD volume, and infused ACD volume was recorded. Each patient's blood was also sampled immediately before and after the TPE procedure. The complete blood count was determined by using the ADVIA 2120i (Siemens, Washington, D.C., USA). This study was carried out with approval from the Institutional Review Board of Yonsei University Health System.
3. Statistics and mathematical formulae
Statistical analysis was performed by using Microsoft Excel 2010 (Microsoft Corporation, Richmond, WA, USA) and Analyse-it (Analyse-it Software Ltd., Leeds, UK). The Shapiro-Wilk test was used to confirm a normal distribution. Pearson's chi-squared test and a two-sample t-test were employed to test statistical significance for demographic characteristics and TPE operational variables. P value<0.05 was considered statistically significant.
To calculate total blood volume (TBV), we used Nadler's equation [
14]:
The formula for plasma removal efficacy (PRE) used by Tormey et al. [
7] was adapted for use in our study. PRE was calculated by using the following formula:
Go to :

RESULTS
1. Plasma exchange performance
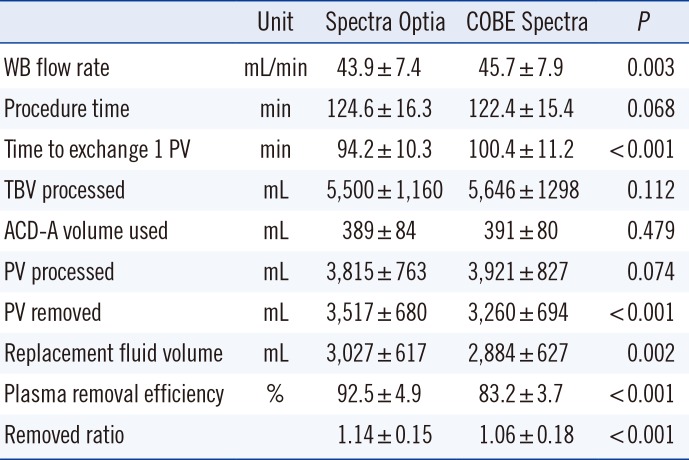
During TPE, 5,500±1,160 mL of plasma were processed in 124.6±16.3 min using the SPO, and 5,646±1,298 mL of plasma were processed in 122.4±15.4 min using the CSP. We compared the time taken to achieve optimal therapeutic exchange of 1 PV between the two systems. The time required to exchange 1 PV was 94.2±10.3 min using the SPO compared with 100.4±11.2 min (
P<0.001) using the CSP. PRE was 92.5±4.9% using the SPO, compared with 83.2±3.7% using the CSP (
P<0.001). The TBV processed, ACD-A volume utilized, and PV processed in both groups showed no statistical differences (
Table 2).
Table 2
Comparison of plasma exchange performance between Spectra Optia and COBE Spectra
|
Unit |
Spectra Optia |
COBE Spectra |
P
|
|
WB flow rate |
mL/min |
43.9±7.4 |
45.7±7.9 |
0.003 |
|
Procedure time |
min |
124.6±16.3 |
122.4±15.4 |
0.068 |
|
Time to exchange 1 PV |
min |
94.2±10.3 |
100.4±11.2 |
<0.001 |
|
TBV processed |
mL |
5,500±1,160 |
5,646±1298 |
0.112 |
|
ACD-A volume used |
mL |
389±84 |
391±80 |
0.479 |
|
PV processed |
mL |
3,815±763 |
3,921±827 |
0.074 |
|
PV removed |
mL |
3,517±680 |
3,260±694 |
<0.001 |
|
Replacement fluid volume |
mL |
3,027±617 |
2,884±627 |
0.002 |
|
Plasma removal efficiency |
% |
92.5±4.9 |
83.2±3.7 |
<0.001 |
|
Removed ratio |
|
1.14±0.15 |
1.06±0.18 |
<0.001 |

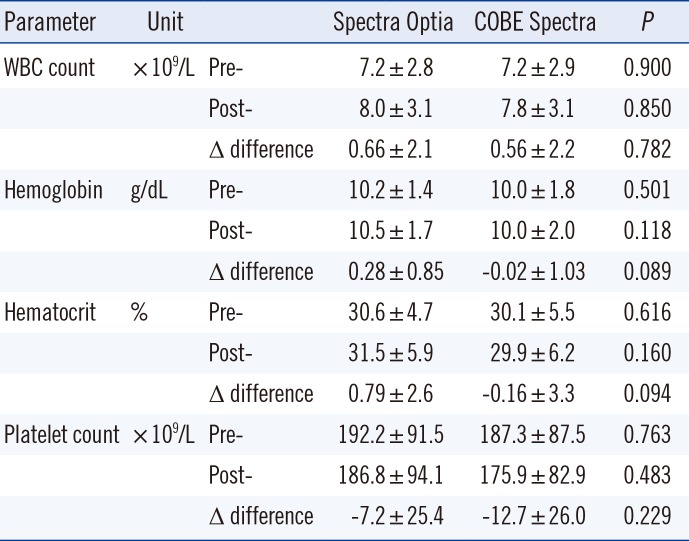
2. Change in hematologic parameters pre- and post-TPE
Both groups showed non-significant post-procedural blood cell component losses, as compared with pre-procedure levels. White blood cell losses were comparable and low with use of both devices; there was a pre- to post-TPE white blood cell count difference of 0.66±2.1 (×10
9/L) with the SPO, and 0.56±2.2 (×10
9/L) using the CSP. Changes in hemoglobin were non-significant (SPO, 0.28±0.85 g/dL; CSP, -0.02±1.03 g/dL). Pre- and post-TPE platelet changes were also non-significant (SPO, -7.2±25.4 [×10
9/L]; CSP, -12.7±26 [×10
9/L]) (
Table 3).
Table 3
Changes of hematologic parameters in patients pre- and post-TPE
|
Parameter |
Unit |
|
Spectra Optia |
COBE Spectra |
P
|
|
WBC count |
× 109/L |
Pre- |
7.2±2.8 |
7.2±2.9 |
0.900 |
|
|
Post- |
8.0±3.1 |
7.8±3.1 |
0.850 |
|
|
Δ difference |
0.66±2.1 |
0.56±2.2 |
0.782 |
|
Hemoglobin |
g/dL |
Pre- |
10.2±1.4 |
10.0±1.8 |
0.501 |
|
|
Post- |
10.5±1.7 |
10.0±2.0 |
0.118 |
|
|
Δ difference |
0.28±0.85 |
-0.02±1.03 |
0.089 |
|
Hematocrit |
% |
Pre- |
30.6±4.7 |
30.1±5.5 |
0.616 |
|
|
Post- |
31.5±5.9 |
29.9±6.2 |
0.160 |
|
|
Δ difference |
0.79±2.6 |
-0.16±3.3 |
0.094 |
|
Platelet count |
× 109/L |
Pre- |
192.2±91.5 |
187.3±87.5 |
0.763 |
|
|
Post- |
186.8±94.1 |
175.9±82.9 |
0.483 |
|
|
Δ difference |
-7.2±25.4 |
-12.7±26.0 |
0.229 |

Go to :

DISCUSSION
The performance of two apheresis devices was compared in the setting of TPE procedures in KT recipients. Because the major goal for TPE is to remove plasma containing pathogenic substances, the time required to exchange 1 PV and PRE are important parameters for evaluating the performance of apheresis devices. Our results suggest that the SPO is more efficient for the removal of plasma, from the standpoint of time required for volume processing.
In addition to performance-related parameters, patient outcomes, such as post-TPE loss of blood cells, are also an important consideration when comparing apheresis devices. Hequet et al. [
8] showed less platelet loss using the SPO after TPE compared with the CSP. Theoretically, a higher PRE may lead to lower platelet loss after TPE. However, our study did not show a statistical difference in platelet loss between the SPO and CSP.
The SPO, unlike the CSP, shows the volume of infused ACD-A to the patient during and after the procedure. During the TPE procedure, the most common side effect is hypocalcemia related to the presence of citrate, which chelates ionized calcium. In our study, the percentage of infused ACD-A volume to used ACD-A volume varied from 5.7% to 31.3%. Therefore, it is useful to predict symptoms of hypocalcemia during the procedure and to decide if additional calcium replacement may be required after the procedure. Additionally, the SPO disposable kit does not require needle spiking to the saline bag, which again makes the SPO superior in terms of infection risk.
Although this study is limited by the retrospective design, we believe that the large number of procedures evaluated in this study compensates for some biases. Our results parallel those of a previous clinical trial that used a non-blinded paired design [
8]. In conclusion, the SPO is superior to the CSP in terms of time required to exchange 1 PV and PRE, has less extracorporeal volume, and is more user-friendly. Therefore, the SPO is a better option over the CSP for TPE procedures in KT patients.
Go to :

Acknowledgments
The authors gratefully acknowledge the excellent work of the apheresis unit staff and all of our colleagues on the kidney transplantation team.
Go to :

Notes
Go to :

References
1. Takahashi K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007; 11:128–141. PMID:
17593512.

2. Jeon BJ, Kim IG, Seong YK, Han BH. Analysis of the results of ABO-incompatible kidney transplantation: In comparison with ABO-compatible kidney transplantation. Korean J Urol. 2010; 51:863–869. PMID:
21221208.

3. Kenmochi T, Saigo K, Maruyama M, Akutsu N, Iwashita C, Otsuki K, et al. Results of kidney transplantation from ABO-incompatible living donors in a single institution. Transplant Proc. 2008; 40:2289–2291. PMID:
18790214.

4. Oettl T, Halter J, Bachmann A, Guerke L, Infanti L, Oertli D, et al. ABO blood group-incompatible living donor kidney transplantation: a prospective, single-centre analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009; 24:298–303. PMID:
18728155.

5. Thielke J, Kaplan B, Benedetti E. The role of ABO-incompatible living donors in kidney transplantation: state of the art. Semin Nephrol. 2007; 27:408–413. PMID:
17616273.

6. Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013; 28:145–284. PMID:
23868759.

7. Tormey CA, Peddinghaus ME, Erickson M, King KE, Cushing MM, Bill J, et al. Improved plasma removal efficiency for therapeutic plasma exchange using a new apheresis platform. Transfusion. 2010; 50:471–477. PMID:
19804570.

8. Hequet O, Stocco V, Assari S, Drillat P, Le QH, Kassir A, et al. Comparison of plasma exchange performances between Spectra Optia and COBE Spectra apheresis systems in repeated procedures considering variability and using specific statistical models. Transfus Apher Sci. 2014; 51:47–53. PMID:
25130725.

9. Yoo S, Lee EY, Huh KH, Kim MS, Kim YS, Kim HO. Role of plasma exchange in ABO-incompatible kidney transplantation. Ann Lab Med. 2012; 32:283–288. PMID:
22779070.

10. Reverberi R, Reverberi L. Removal kinetics of therapeutic apheresis. Blood Transfus. 2007; 5:164–174. PMID:
19204770.
11. Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. J Clin Apher. 2007; 22:270–276. PMID:
17722046.

12. De Silvestro G, Marson P, Russo GE, Vicarioto M, Donadel C. National survey of apheresis activity in Italy (2000). Transfus Apher Sci. 2004; 30:61–71. PMID:
14746823.

13. Norda R, Stegmayr BG. Swedish Apheresis Group. Therapeutic apheresis in Sweden: update of epidemiology and adverse events. Transfus Apher Sci. 2003; 29:159–166. PMID:
12941356.

14. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962; 51:224–232. PMID:
21936146.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download