Dear Editor
We report an unusual case of myeloperoxidase (MPO)-positive acute megakaryoblastic leukemia (AMKL). The patient was a 63-yr-old woman referred to our hospital with anemia and dyspnea. General physical examination revealed no lymphadenopathy or hepatosplenomegaly. An initial complete blood count indicated white blood cell counts of 26.78×109/L, including 47% blasts, hemoglobin of 7.2 g/dL, and platelets of 519×109/L. A peripheral blood smear showed blasts with prominent nucleoli and blue cytoplasm. Some giant platelets were also observed. A bone marrow (BM) biopsy showed hypercellularity with no fibrosis, packed with blasts, and revealed an increase in megakaryocyte number. BM aspirates showed that blasts accounted for 70% of all nucleated elements. Most blasts had medium-to-large, finely chromatinated nuclei with distinct nucleoli. Some showed multiple clear cytoplasmic projections (Fig. 1A, B) or Auer rods. No dysplastic features of hematologic precursors were observed. Cytochemical stain showed some blasts were MPO-positive (Fig. 1C) but negative for periodic acid-Schiff (PAS) stain and non-specific esterase (NSE, α-naphthyl butyrate).
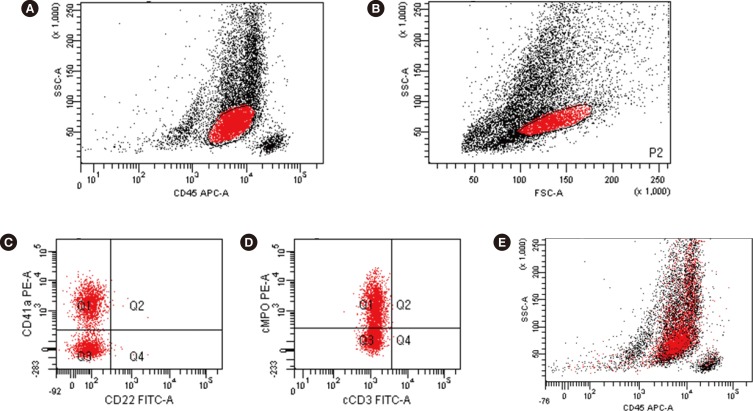
Immunophenotyping showed a distinct population in the CD45 vs. SSC plot that was positive for CD7, CD11c, CD13, CD33, CD41a, CD117, cytoplasmic MPO, and HLA-DR and negative for CD2, CD3, CD5, CD10, CD14, CD19, CD20, CD22, CD34, CD56, CD64, and cytoplasmic CD79a. About 57.0% of blasts were CD41a-positive, and 57.7% were cytoplasmic MPO-positive. A blast population co-expressing CD41a and cytoplasmic-MPO was also observed (Fig. 2). Conventional karyotyping revealed a 46,XX karyotype. Molecular studies did not reveal any genetic abnormalities. As the patient refused treatment, only supportive care was provided, and the patient died from pneumonia four months later.
Diagnosis of AMKL relies on multiple criteria including morphology, immunophenotyping, cytochemical stain, and ultrastructural studies [1]. The standard for diagnosis is demonstration of platelet glycoprotein (GP)-CD41 (GP IIb/IIIa) and/or CD61 (GP IIIa) by immunophenotyping. Cytoplasmic expression is more specific owing to possible contamination of platelets [2]. In cytochemical stain, megakaryoblasts are not reactive with MPO or Sudan Black B. Cells of the megakaryocytic lineage are usually positive in PAS stain owing to glycogen granules in the cytoplasm, and they are typically present in the periphery on stain, with prominent cytoplasmic blebs [3]. Reactivity with α-naphthyl acetate, but not with α-naphthyl butyrate, a different substrate for NSE, is characteristic of megakaryoblasts [4]. An ultrastructural platelet peroxidase reaction by cytochemistry, while difficult to perform, is also diagnostic for megakaryoblasts [1]. Megakaryoblasts also show acid phosphatase reactivity localizing to the Golgi [4]. There are no distinct chromosomal abnormalities, but inv(3)(q21q26.2) and t(3:3)(q21q26.2) are associated with megakaryocytic/megakaryoblastic differentiation [2].
Although MPO is an exclusive marker for leukemia of the megakaryoblastic lineage, few MPO-positive AMKL cases have been reported [5,6,7]. Park et al. [5] reported one Korean case in 1996, where blasts showed morphology typical of megakaryoblasts, and CD61 expression was confirmed by flow cytometry. The blasts also were weakly positive for MPO in cytochemical stain, and some Auer rods were observed. The sample was PAS-negative, but positive foci in the Golgi were observed with NSE stain (α-naphthyl acetate). Tallman et al. [6] reviewed 20 patients previously diagnosed as having AMKL using morphologic evidence and found two MPO-positive patients. One patient expressed factor VIII, a megakaryocytic lineage marker, and karyotyping revealed t(3:3)(q21;q26). The other patient had a normal karyotype and no evidence of the megakaryocytic lineage. In another study, AMKL was diagnosed by using another platelet marker, CD31, with MPO [7]. CD31, also known as PECAM-1 (platelet endothelial cell adhesion molecule 1), is a 130-kDa transmembrane glycoprotein on the surface of platelets, monocytes, macrophages, and neutrophils [8]. Immunolocalization of CD31 is limited to megakaryocytes in normal BM or in cases of myelofibrosis [9]. A failed BM aspiration prevented analysis by flow cytometry. The sample was negative for factor VIII on immunohistochemistry. However, the authors diagnosed the case as AMKL on the basis of typical blast morphology and positive immunohistochemical reactivity for CD31, CD43, and MPO in the BM biopsy [7].
In this case, blasts showed typical megakaryoblastic morphology with some Auer rods. Unlike most cases, the blasts co-expressed CD41a and cytoplasmic-MPO, were MPO-positive on cytochemical stain, and PAS-negative. According to the 2008 WHO classification, megakaryocytic lineage markers are not included in the diagnosis of mixed phenotype acute leukemia [10]. On the basis of previous reports, we conclude that this was an MPO-positive AMKL. AMKL is rare, and its diagnosis is not clearly defined compared with other types of AML. Morphologic evidence is still important, and comprehensive analyses are required when diagnosing acute leukemia, especially with a megakaryocytic lineage.
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Mathur NB, Joshi N, Singh T, Singh M. Congenital acute megakaryocytic leukemia. Indian J Med Paediatr Oncol. 2011; 32:165–167. PMID: 22557786.

2. Arber DA, Brunning RD, Orazi A, Porwit A, Peterson L, Thiele J. Acute myeloid leukemia, not otherwise specified. In : Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008. p. 130–139.
3. Sun T, editor. Flow cytometry, immunohistochemistry, and molecular genetics for hematologic neoplasms. 2012. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;2012. p. 150–152.
4. Pombo De Oliveira MS, Gregory C, Matutes E, Parreira A, Catovsky D. Cytochemical profile of megakaryoblastic leukaemia: a study with cytochemical methods, monoclonal antibodies, and ultrastructural cytochemistry. J Clin Pathol. 1987; 40:663–669. PMID: 3038965.

5. Park CJ, Cho HC, Park YS. A case of acute megakaryoblastic leukemia showing Aure rods. Korean J Hematol. 1996; 31:161–165.
6. Tallman MS, Neuberg D, Bennett JM, Francois CJ, Paietta E, Wiernik PH, et al. Acute megakaryocytic leukemia: the Eastern Cooperative Oncology Group experience. Blood. 2000; 96:2405–2411. PMID: 11001891.
7. Majhi U, Murhekar K, Sundersingh S, Rajalekshmi KR. Megakaryoblastic leukemia presenting as pancytopenia and extensive myelofibrosis in a child diagnosed by myeloid markers and CD 31. Indian J Med Paediatr Oncol. 2012; 33:59–61. PMID: 22754213.

8. Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006; 54:385–395. PMID: 16234507.

9. Calapso P, Vitarelli E, Crisafulli C, Tuccari G. Immunocytochemical detection of megakaryocytes by endothelial markers: a comparative study. Pathologica. 1992; 84:215–223. PMID: 1437309.
10. Borowitz MJ, Bene MC, Harris NL, Porwit A, Matutes E. Acute leukemias of ambiguous lineage. In : Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008. p. 150–155.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download