Staphylococcus aureus bacteremias (SAB), the most common cause of nosocomial bacteremias, are associated with high mortality and morbidity [
1]. Methicillin-resistant
S. aureus (MRSA) bacteremia is characterized by higher mortality than methicillin-susceptible
S. aureus (MSSA) bacteremia. Thus, as a precaution in the absence of an early, definitive method to detect MRSA, the first-line treatment for nosocomial SAB is vancomycin [
1]. However, vancomycin is associated with a higher mortality rate than β-lactams in the treatment of MSSA bacteremia [
2]. Thus, rapid identification of
S. aureus and determination of methicillin susceptibility are of crucial importance [
3]. Conventional diagnosis of SAB requires at least 2-3 days [
4]. Mass spectrometric and molecular tools, performed directly on positive blood cultures (BCs), enable diagnoses in less than 4 hr [
5,
6,
7]. However, these new tools are not yet available in every laboratory.
This study evaluated the accuracy of two immunochromatographic tests (ICT) that may be used directly in BCs: Binax Now S. aureus (BNSA) for S. aureus identification and Binax Now PBP2a (BNPBP2a; Alere SAS, Jouy-en-Josas, France) for determining methicillin resistance. Tests were performed by following the manufacturer's instructions. One hundred colony forming units of 17 MRSA strains, including the 15 primary worldwide MRSA clones, sequence type (ST)1, ST5 (n=2), ST8 (n=2), ST22, ST30, ST45, ST59, ST72, ST80, ST88, ST93, ST228, ST239, ST247, and ST398 clones (French National Reference Center, Lyon, France), were inoculated into 10 mL of fresh human blood from healthy volunteers in charcoal aerobic (FA) and non-charcoal anaerobic (SN) Bact/ALERT BC bottles (bioMérieux, Marcy l'Etoile, France). Following the detection of growth with the 3D Bact/ALERT instrument, a direct examination was performed, and each bottle showing Gram positive cocci in cluster aggregations (GPCCA) was tested with the BNSA test followed by the BNPBP2a test. The BNSA test was positive in 17/17 and 16/17 SN and FA BC bottles, respectively. The single strain that tested negative in FA bottles, an ST45 strain, was positive on retesting. Therefore, the first test was considered as a technical error. The BNPBP2a test was positive in 17/17 and 17/17 of SN and FA BC bottles, respectively.
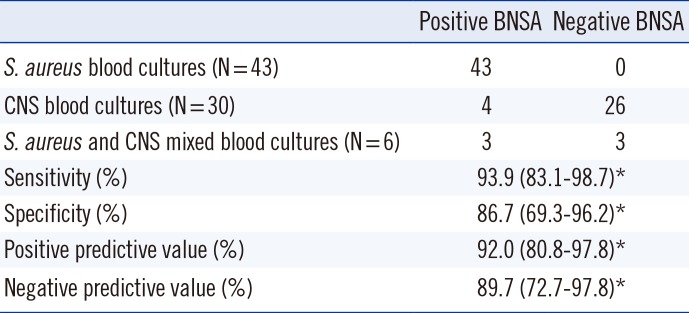
Next, blood from 60 patients (23 females and 37 males, mean age of 41.6 yr) that were hospitalized in surgical and medical care units of the Hospices Civils de Lyon were prospectively collected in accordance with the ethical board of our institution. To reduce the rate of BC positive to coagulase-negative staphylococci, BC bottles from patients hospitalized (i) for more than 24 hr and (ii) in care units known to have a high rate of BC with coagulase-negative staphylococci (CNS) were excluded. Samples were cultured in 79 bottles and then evaluated from August to December 2010. The inclusion criteria for BCs included a growth-detection time of less than 25 hr and the detection of GPCCA by microscopic examination. ICTs were performed on 38 non-charcoal aerobic (SA) and 41 charcoal anaerobic (FN) bottles within 4 hr of growth detection, and the results were compared with species identification by matrix assisted laser desorption ionization/time of flight mass spectrometry (MALDI-TOF MS) by the Saramis system (bioMérieux, Marcy L'Etoile, France) and with methicillin susceptibility testing by the Phoenix® system (BD, Pont de Claix, France) using subcultures on blood agar plates. Any discrepancy in methicillin susceptibility results was checked by PCR for the presence of the mecA gene. Of the 79 BC bottles tested, 73 yielded a mono-microbial culture, and four out of these 73 bottles yielded false positive BNSA results. All four were from charcoal bottles (
Table 1). Whereas BNSA testing on Bact/ALERT® bottles was cleared by the FDA [
8], this work is the first external study evaluating the combination of BNSA and Bact/ALERT bottles, including some charcoal bottles. As shown in
Fig. 1, these four false positive BNSA tests exhibited the expected pink control band and a very low intensity gray color band for the sample. These four bottle samples (three
S. epidemidis and one
S. capitis) were retested, and while three showed similar low positive results, one was clearly negative. The gray color of the false positive tests and the lack of similar reports from testing BC bottles without charcoal, may suggest interference with charcoal particles as a cause of the false positives. In contrast, no indeterminate result was obtained with the BNPBP2a test using a filtration protocol instead of centrifugation for the charcoal bottles. Therefore, the filtration protocol seemed to be more appropriate than differential centrifugation, and the manufacturer's protocol should be revised to recommend use of a filtration method or recommending against the use of charcoal-containing media with the BNSA test. Of the six mixed SA/CNS BC, three tested negative for
S. aureus using BNSA, suggesting the inoculum was too low. Similar false negative BNSA tests were reported by Dhilman
et al. [
9] and Yossepowitch
et al. [
10], with 2/2 and 4/5 false negative results, respectively, found for mixed SA/CNS BC. False negative results for mixed SA/CNS BC were also observed with molecular testing [
6,
11]. Our BNSA results were both less sensitive and less specific than those reported by Qian
et al. [
12] (sensitivity [Se]: 97.6%; specificity [Sp]: 100%) and Dhiman
et al. [
9] (Se: 95.8%; Sp: 99.6%), probably due to their use of charcoal-free BC bottles, Bactec and VersaTREK, respectively.
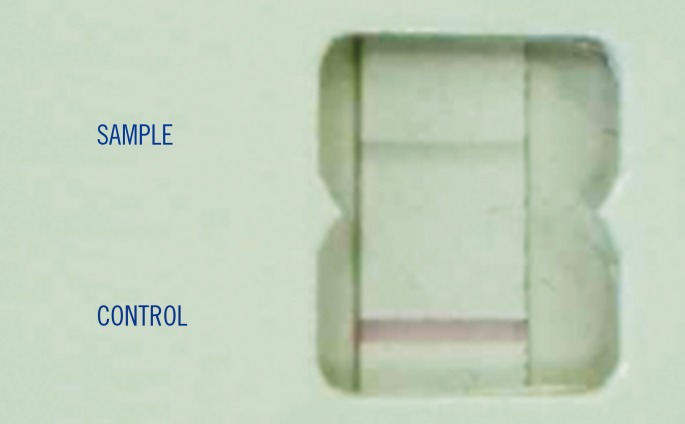 | Fig. 1Example of false positive Binax Now Staphylococcus aureus test with charcoal particles in Bact/ALERT bottles.
|
Table 1
Results and performance of the Binax Now Staphylococcus aureus tests performed on 79 positive blood cultures with results from direct microscopic examination of Gram positive cocci arranged in cluster aggregations
|
Positive BNSA |
Negative BNSA |
|
S. aureus blood cultures (N=43) |
43 |
0 |
|
CNS blood cultures (N=30) |
4 |
26 |
|
S. aureus and CNS mixed blood cultures (N=6) |
3 |
3 |
|
Sensitivity (%) |
93.9 (83.1-98.7)*
|
|
Specificity (%) |
86.7 (69.3-96.2)*
|
|
Positive predictive value (%) |
92.0 (80.8-97.8)*
|
|
Negative predictive value (%) |
89.7 (72.7-97.8)*
|

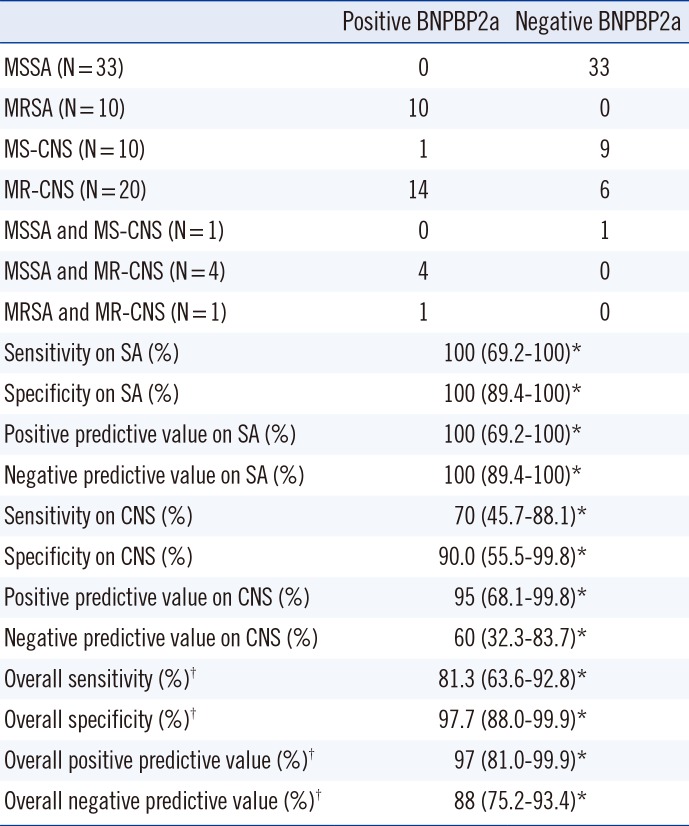
Consistent with studies by Romero-Gomez
et al. [
4] and Montgomery (21st European Congress of Clinical Microbiology and Infectious Diseases, abstract P1040), BNPBP2a detected 10 MRSA strains among the 43
S. aureus BCs (Se: 100%; Sp: 100%;
Table 2). Both false positive and false negative results were obtained when detecting methicillin resistance in CNS BCs; however, this test was not designed for CNS strains. Surprisingly, of the six mixed SA/CNS BCs, BNPBP2a testing showed concordant results with the reference method. The sole false positive BNPBP2a test observed with CNS was negative on retesting, suggesting that the first result was an artifact of the procedure. Conversely, false negative results in the detection of methicillin resistance in colonies was previously reported with the ClearView PBP2a ICT and with the Slidex MRSA detection test [
13]. To circumvent this lack of sensitivity, two different protocols have been reported: the use of an increased inoculum density or the use of colonies previously induced either by cefoxitin or oxacillin to produce PBP2a [
13]. Thus, additional studies are required to determine, if these protocols could be applied to the BNPBP2a test.
Table 2
Results and performance of Binax Now PBP2a tests performed on 79 positive blood cultures with results from direct microscopic examination of Gram positive cocci arranged in cluster aggregations
|
Positive BNPBP2a |
Negative BNPBP2a |
|
MSSA (N=33) |
0 |
33 |
|
MRSA (N=10) |
10 |
0 |
|
MS-CNS (N=10) |
1 |
9 |
|
MR-CNS (N=20) |
14 |
6 |
|
MSSA and MS-CNS (N=1) |
0 |
1 |
|
MSSA and MR-CNS (N=4) |
4 |
0 |
|
MRSA and MR-CNS (N=1) |
1 |
0 |
|
Sensitivity on SA (%) |
100 (69.2-100)*
|
|
Specificity on SA (%) |
100 (89.4-100)*
|
|
Positive predictive value on SA (%) |
100 (69.2-100)*
|
|
Negative predictive value on SA (%) |
100 (89.4-100)*
|
|
Sensitivity on CNS (%) |
70 (45.7-88.1)*
|
|
Specificity on CNS (%) |
90.0 (55.5-99.8)*
|
|
Positive predictive value on CNS (%) |
95 (68.1-99.8)*
|
|
Negative predictive value on CNS (%) |
60 (32.3-83.7)*
|
|
Overall sensitivity (%)†
|
81.3 (63.6-92.8)*
|
|
Overall specificity (%)†
|
97.7 (88.0-99.9)*
|
|
Overall positive predictive value (%)†
|
97 (81.0-99.9)*
|
|
Overall negative predictive value (%)†
|
88 (75.2-93.4)*
|

Although our study has some limitations due to the limited number of staphylococcal BCs (n=79) and the lack of methicillin resistant strains exhibiting the very rare and newly described
mecC gene [
14], these ICT methods performed well in rapidly detecting
S. aureus/CNS and MSSA/MRSA. A positive BNSA test result could be transmitted to clinicians as "
S. aureus," whereas a negative result must be transmitted only as "negative test," in association with results of direct examination of blood culture. A negative test could be due to CNS as well as other Gram positive bacteria in clusters (e.g.,
Micrococcus sp.). Similarly, the BNPBP2a results must be limited to a MSSA/MRSA distinction. In comparison, probes using peptide nucleic acid fluorescence
in situ hybridization technology (PNA-FISH) have enabled the differentiation of
S. aureus and CNS in less than 15 min, with a sensitivity of 100% and a specificity of 98.5%, but do not allow for the detection of methicillin resistance [
5].
Molecular tests have also been widely evaluated. The Genotype Gram positive test enables the identification of some strains of
Staphylococcus, and detects
mecA with accuracy in 5 hr [
15]. The GeneOhm assay shows better sensitivity (100%) and specificity (100%) for
S. aureus detection than BNSA, whereas BNPBP2a shows better results for MRSA detection than GeneOhm, most likely due to the SCC
mec-positive strains that do not express
mecA [
6]. The fully automated Xpert MRSA/SA BC test is better for
S. aureus detection (Se: 100%; Sp: 98.6%) but not for the detection of MRSA (Se: 98.3%; Sp: 99.4%) when compared with BNSA and BNPBP2a results, respectively [
11]. The MALDI-TOF MS technique can identify
S. aureus and CNS directly from BC in 94% to 100% and 25% to 100% of cases, respectively, depending on the CNS species, pre-analytical methods, and type of BC bottles that are included in the process [
7]. However, thus far, MALDI-TOF MS cannot detect methicillin resistance [
16]. An alternative method may be the combination of MALDI-TOF MS for the identification of
S. aureus and a BNPBP2a test to determine methicillin resistance.
Finally, diagnosis of staphylococcal bacteremia may be hastened with all of these new tools; however, molecular tests are expensive [
5] and MALDI-TOF MS, which is less expensive, does not enable methicillin resistance determination [
7]. Thus, the combination of BNSA and BNPBP2a tests appear to be an efficient diagnostic strategy, because they are simple, rapid, and feasible for any laboratory personnel.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download