Eosinophilia, defined as an absolute eosinophil count of >0.45 ×10
9/L in peripheral blood, occurs in allergic diseases, parasitic infections, and cancer [
1]. Recently, toxocariasis has emerged as a major cause of eosinophilia and induced eosinophilic infiltration in internal organs [
2]. Toxocariasis caused by infection with
Toxocara canis larvae occurs by accidental ingestion of embryonated eggs or larvae from a range of wild and domestic animals [
3]. Many epidemiological studies have been performed worldwide, but most have focused on schoolchildren who are at higher risk of infection because of their play habits and typically poor hygiene [
4,
5,
6]. Although less common, humans can also become infected if they eat undercooked meat from an animal infected with
T. canis larvae [
7]. Especially in Korea, it is thought that toxocariasis is likely to be more prevalent owing to the habitual intake of raw liver [
2,
8,
9]. Although the epidemiology of toxocariasis is affected by regional culture, the epidemiology of toxocariasis in the regions of Gwangju and Jeonnam-province has not been evaluated. We investigated the seroepidemiological, clinical, and laboratory characteristics of patients suspected to have toxocariasis in Gwangju and Jeonnam-province, Korea.
The medical records of 228 patients whose specimens were submitted for anti-
T. canis IgG testing were analyzed retrospectively at Chonnam National University Hospital and Chonnam National University Hwasun Hospital from 2010 to 2012. Levels of specific IgG to
Toxocara excretory/secretory antigen were measured by using
T. canis ELISA kit (Bordier Affinity Products, Crissier, Switzerland) [
10]. The investigated variables were age, sex, laboratory parameters such as complete blood count, liver function tests, renal function tests, total IgE, eosinophil cationic protein (ECP) levels, and history or presence of i) asthma or rhinitis, ii) cancer, iii) drug use, iv) freshwater fish, raw meat, or raw cow liver consumption, v) organ involvement (liver or lung), and vi) other parasitic infections (clonorchiasis, paragonimiasis, cysticercosis, or sparganosis).
Chi-square or Fisher exact test was performed to determine the distributions of categorical variables, including sex and other risk factors between groups (non-eosinophilic vs. eosinophilic group; seronegative vs. seropositive group; and organ involvement (-) vs. organ involvement (+) group). Student t-tests were used to compare continuous variables, such as age and laboratory parameters. The likelihood-ratio chi-square was used to calculate the odds ratio (OR) for eosinophilia, seropositivity, and organ involvement. Logistic regression analysis was usedfor multivariate analysis. Spearman correlation coefficients were used to examine relationships between the optical density levels of anti-
T. canis IgG and the eosinophil counts or duration of eosinophilia. The correlation coefficients (r-values) were interpreted by Dancey and Reidy's categorization [
11]. Here, r-value of ±1 is interpreted as a perfect correlation; ±0.7 to ±0.9 as strong; ±0.4 to ±0.6 as moderate; ±0.1 to ±0.3 as weak correlation; and an r-value of 0 as zero correlation, implying no correlation.
P value <0.05 indicated significance for all analyses. All statistical analyses were performed by using PASW version 18.0 (SPSS Inc., Chicago, IL, USA).
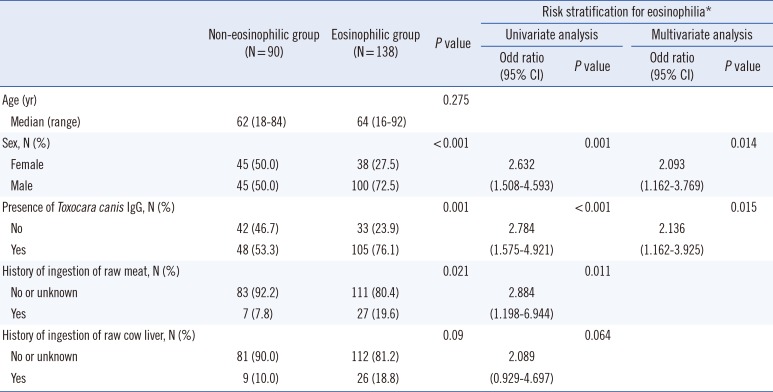
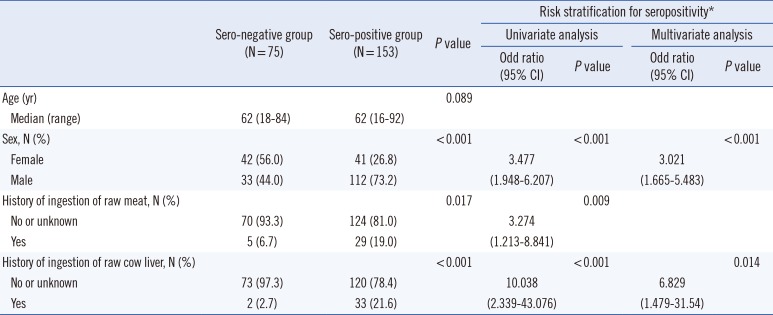
Tables 1 and
2 describe the clinical characteristics and their risk stratifications according to eosinophilia and toxocariasis seropositivity. Male sex was predominant in both eosinophilic and seropositive groups (72.5% and 73.2%, both
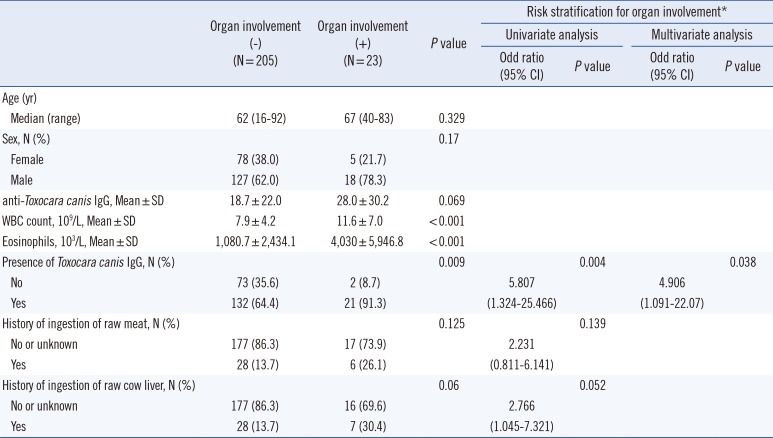
P<0.001). The general seroprevalence was 67.1% (153/228), and the seropositive rates in the eosinophilic and non-eosinophilic groups were 76.1% and 53.3%, respectively. Raw meat or raw cow liver ingestion occurred more frequently in the eosinophilic group (OR=2.884 and 2.089, respectively) and the seropositive group (OR=3.274 and 10.038, respectively) than in their counterparts. Other causes, including histories of other parasitic infections, asthma or allergic rhinitis, cancer, drug use, and fresh water fish ingestion, were not significantly related to eosinophilia or seropositivity. Of the 228 patients, 23 showed organ involvement findings in radiological evaluations. The presence of organ involvement was associated with seropositivity (OR=5.807) and a history of consuming raw cow liver (OR=2.766;
Table 3). Multivariate analysis revealed that male and seropositivity were the independent risk factors for eosinophilia, male and the history consuming raw cow liver for seropositivity, and seropositivity for organ involvement, respectively. Patients with organ involvement had higher eosinophil levels and white blood cell counts (
P< 0.001, both) than patients without organ involvement. Spearman correlation analysis indicated that the optical density levels of IgG showed a weak correlation with eosinophil counts (r=0.234,
P<0.001) and the duration of eosinophilia (r=0.155,
P=0.019;
Supplemental Data Figure S1). The eosinophil counts alone also showed a moderate correlation with the duration of eosinophilia (r=0.585,
P<0.001; data not shown).
Table 1
Clinical characteristics of patients and risk stratifications according to eosinophilia
|
Non-eosinophilic group (N=90) |
Eosinophilic group (N=138) |
P value |
Risk stratification for eosinophilia*
|
|
Univariate analysis |
Multivariate analysis |
|
Odd ratio (95% CI) |
P value |
Odd ratio (95% CI) |
P value |
|
Age (yr) |
|
|
0.275 |
|
|
|
|
|
Median (range) |
62 (18-84) |
64 (16-92) |
|
Sex, N (%) |
|
|
<0.001 |
2.632 (1.508-4.593) |
0.001 |
2.093 (1.162-3.769) |
0.014 |
|
Female |
45 (50.0) |
38 (27.5) |
|
Male |
45 (50.0) |
100 (72.5) |
|
Presence of Toxocara canis IgG, N (%) |
|
|
0.001 |
2.784 (1.575-4.921) |
< 0.001 |
2.136 (1.162-3.925) |
0.015 |
|
No |
42 (46.7) |
33 (23.9) |
|
Yes |
48 (53.3) |
105 (76.1) |
|
History of ingestion of raw meat, N (%) |
|
|
0.021 |
2.884 (1.198-6.944) |
0.011 |
|
|
|
No or unknown |
83 (92.2) |
111 (80.4) |
|
Yes |
7 (7.8) |
27 (19.6) |
|
History of ingestion of raw cow liver, N (%) |
|
|
0.09 |
2.089 (0.929-4.697) |
0.064 |
|
|
|
No or unknown |
81 (90.0) |
112 (81.2) |
|
Yes |
9 (10.0) |
26 (18.8) |

Table 2
Clinical characteristics of patients and risk stratifications according to seropositivity for toxocariasis
|
Sero-negative group (N=75) |
Sero-positive group (N=153) |
P value |
Risk stratification for seropositivity*
|
|
Univariate analysis |
Multivariate analysis |
|
Odd ratio (95% CI) |
P value |
Odd ratio (95% CI) |
P value |
|
Age (yr) |
|
|
0.089 |
|
|
|
|
|
Median (range) |
62 (18-84) |
62 (16-92) |
|
Sex, N (%) |
|
|
< 0.001 |
3.477 (1.948-6.207) |
< 0.001 |
3.021 (1.665-5.483) |
< 0.001 |
|
Female |
42 (56.0) |
41 (26.8) |
|
Male |
33 (44.0) |
112 (73.2) |
|
History of ingestion of raw meat, N (%) |
|
|
0.017 |
3.274 (1.213-8.841) |
0.009 |
|
|
|
No or unknown |
70 (93.3) |
124 (81.0) |
|
Yes |
5 (6.7) |
29 (19.0) |
|
History of ingestion of raw cow liver, N (%) |
|
|
< 0.001 |
10.038 (2.339-43.076) |
< 0.001 |
6.829 (1.479-31.54) |
0.014 |
|
No or unknown |
73 (97.3) |
120 (78.4) |
|
Yes |
2 (2.7) |
33 (21.6) |

Table 3
Analysis of clinical and laboratory characteristics for the risk of organ involvement
|
Organ involvement
(-)
(N=205) |
Organ involvement
(+)
(N=23) |
P value |
Risk stratification for organ involvement*
|
|
Univariate analysis |
Multivariate analysis |
|
Odd ratio (95% CI) |
P value |
Odd ratio (95% CI) |
P value |
|
Age (yr) |
|
|
|
|
|
|
|
|
Median (range) |
62 (16-92) |
67 (40-83) |
0.329 |
|
Sex, N (%) |
|
|
0.17 |
|
|
|
|
|
Female |
78 (38.0) |
5 (21.7) |
|
Male |
127 (62.0) |
18 (78.3) |
|
anti-Toxocara canis IgG, Mean ± SD |
18.7 ± 22.0 |
28.0 ± 30.2 |
0.069 |
|
|
|
|
|
WBC count, 109/L, Mean ± SD |
7.9 ± 4.2 |
11.6 ± 7.0 |
<0.001 |
|
Eosinophils, 103/L, Mean ± SD |
1,080.7 ± 2,434.1 |
4,030 ± 5,946.8 |
<0.001 |
|
Presence of Toxocara canis IgG, N (%) |
|
|
0.009 |
5.807 (1.324-25.466) |
0.004 |
4.906 (1.091-22.07) |
0.038 |
|
No |
73 (35.6) |
2 (8.7) |
|
Yes |
132 (64.4) |
21 (91.3) |
|
History of ingestion of raw meat, N (%) |
|
|
0.125 |
2.231 (0.811-6.141) |
0.139 |
|
|
|
No or unknown |
177 (86.3) |
17 (73.9) |
|
Yes |
28 (13.7) |
6 (26.1) |
|
History of ingestion of raw cow liver, N (%) |
|
|
0.06 |
2.766 (1.045-7.321) |
0.052 |
|
|
|
No or unknown |
177 (86.3) |
16 (69.6) |
|
Yes |
28 (13.7) |
7 (30.4) |

Toxocariasis has been recognized as the most commonly neglected parasitic infection in the United States, and its global importance might be greatly underestimated [
12]. Although a nationwide survey has not been performed in Korea, regional studies enable estimates of the prevalence of toxocariasis as follows (
Supplemental Data Table S1): 86.7% (Seoul), 68.0% (Seoul), 62.1% (Pohang), and 45.5% (Chungnam-province) [
2,
8,
9,
13]. A previous study reported that the seroprevalence in healthy rural Korean adults might be around 5% [
14], but this study showed 53.3% seroprevalence in the non-eosinophilic group. This might be because many healthy subjects display residual antibodies and the IgG assay cannot rule out past infection within the non-eosinophilic group. Thus, specific IgE for
T. canis would be useful for differentiating acute and past infections, although it is not widely used in clinical laboratories because a commercial kit is not yet available [
15]. The cross-reactivity to other helminthic infections should be considered a factor for high seroprevalence in this study [
10]. We could not find any evidence of filariasis in this study but this is limited owing to the retrospective nature of our study. Although filariasis is well controlled in Korea [
16], the potential cross-reactivity should be considered in the clinical use of this ELISA kit.
It is noteworthy that seropositivity may be an independent risk factor for eosinophilia. Considering the high prevalence of toxocariasis in eosinophilic patients, a diagnostic approach to toxocariasis should be performed with a view to diagnose idiopathic hypereosinophilic syndrome [
17]. Male sex is considered a risk factor for both eosinophilia and toxocariasis; this is likely because of the habit of ingesting raw meat [
2]. This study also showed that the ingestion of raw cow liver substantially increased the risk of toxocariasis, more than 10 times, which is in line with other studies [
2,
8,
9]. A recent meta-analysis found that a higher prevalence of
T. canis infection was associated with asthma [
18], but we could not find significant association between seropositivity and asthma. In addition to asthma, other causes of eosinophilia such as other allergic diseases, underlying cancer, and other parasitic infections should also be ruled out for the evaluation of eosinophilia. We found that 57% (25/44) of eosinophilic patients, seronegative for toxocariasis, had evidence of allergic diseases, underlying cancer, or other parasitic infections.
Almost all patients with organ involvement were seropositive for toxocariasis, except for two patients diagnosed as having paragonimiasis and the Drug Reaction with Eosinophilia and Systemic Symptom syndrome. This was suggestive of the phenomenon of visceral larva migrans, because the lesions resolved or moved on follow-up imaging. These findings highlight that clinicians should be aware of toxocariasis serology in eosinophilic patients with organ involvement [
19]. High anti-
T. canis IgG levels also correlated with high eosinophil counts and persistent eosinophilia. This supports the need for proper treatment of toxocariasis to control the levels of anti-
T. canis IgG associated with eosinophilia-related symptoms in these patients. In addition, anti-
T. canis IgG levels seemed to diminish after six months when we followed up serially. Especially in two patients, seroconversion from positive to gray zone occurred after 21 months (data not shown). Although the duration of seropositivity for
T. canis is not well known, serial follow-up would be recommended with an interval of more than six months.
This study had some limitations. The clinical, laboratory parameters might not have been fully investigated in all patients, because this study was performed retrospectively. ECP and total IgE levels have been thought to be markers for toxocariasis [
20], but we did not demonstrate correlations with eosinophilia or toxocariasis. In particular, 120 of 153 seropositive patients had no evidence of raw cow liver consumption. It seems likely that history taking for risk factors may had been performed incompletely. Thus, thorough evaluation with laboratory parameters and history taking should be done when toxocariasis is suspected. Regretfully, the current data may not reflect the seroprevalence of toxocariasis exactly, because the sample population included patients with suspected toxocariasis at two university hospitals. Although regional data are used in this study, these data are consistent with other Korean data for the epidemiology of toxocariasis in eosinophilic patients. The current study highlights that toxocariasis is a reasonable focus as a cause of eosinophilia and is associated with organ involvement. A serological evaluation for toxocariasis is essential for patients with eosinophilia, considering the cultural habit of ingesting raw cow liver in Korea.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download