Abstract
Background
The therapeutic efficacy of red blood cell (RBC) transfusions in patients with autoimmune hemolytic anemia (AIHA) is highly debated because of speculations on the increased risk of transfusion reactions; yet it is a suggested adjuvant therapy in anemic patients with life-threatening hypoxemia. In this study, we evaluated the safety and efficacy of RBC transfusions in AIHA patients.
Methods
Daily changes in hemoglobin, total bilirubin, and lactate dehydrogenase (LDH) were assessed in 161 AIHA patients without bleeding history who were transfused once with 1-5 units of the least-incompatible RBCs and monitored over a seven-day period. Post-transfusion patients positive for alloantibodies only or those without RBC-specific antibodies were considered as control groups (N=100 for both groups).
Results
The three groups revealed similar increases in hemoglobin of 1.40-1.70 g/dL (autoantibodies), 1.20-1.60 g/dL (alloantibodies only), and 1.40-1.55 g/dL (no antibodies) for seven days following transfusion of 10 mL RBCs/kg. During follow-up, no significant changes in total bilirubin or LDH levels were detected in the AIHA group compared with controls. Influences due to autoantibody type, direct antiglobulin test (DAT) specificity and strength, and steroid therapy status on transfusion reactions were not evident in AIHA patients. In addition, changes in hemoglobin levels were significantly higher (P<0.001) in severe anemia (<5 g/dL) than in other patients.
The diagnosis of autoimmune hemolytic anemia (AIHA) is made on the basis of positive direct antiglobulin test (DAT) and a serologic evaluation demonstrating the presence of autoantibodies specific to the patient's red blood cells (RBCs) [1, 2, 3, 4, 5]. The feasibility of blood transfusion in AIHA patients is heavily debated because of difficulties in crossmatching and the increased risk of transfusion reactions, since transfused RBCs may be destroyed more rapidly in patients with active hemolysis, which can result in hemoglobinemia, hemoglobinuria, and subsequent disseminated intravascular coagulopathy. Therefore, clinicians must exercise extreme caution when deciding transfusion in AIHA patients, and then should use only the smallest volume of blood necessary to alleviate life-threatening conditions [6, 7].
Conversely, RBC transfusion has been suggested as an adjunctive therapeutic option in patients with autoantibodies despite the risk of hemolysis [8]. This is based on the belief that the increased oxygen-carrying capacity provided by the transfused RBCs may satisfy a patient's need until other treatment options become effective, particularly in those with symptomatic cardiovascular diseases for which sufficient oxygen supply is important [1, 4, 9]. However, data weighing the safety and efficacy of transfusions in patients with AIHA is scarce and should be further investigated. In this study, we aimed to evaluate whether transfusion of the least-incompatible blood was sufficient to increase hemoglobin levels without causing a significant increase in risk of hemolysis in patients with AIHA.
Total 161 anemic patients with positive DAT and autologous control panel antibody identification tests that received one transfusion of 1-5 units (320 mL or 400 mL) of the least-incompatible packed RBCs and monitored over a period of seven days from January 2006 to March 2011 at Asan Medical Center were initially selected as a patient group.
DATs were performed by using the gel-based Diamed-ID system (Bio-Rad Laboratories, Hercules, CA, USA). Antibody identification testing was performed with Diamed-ID-Card LISS/Coombs+Enzyme Test (Bio-Rad Laboratories). Autoantibodies were classified into three groups (warm-reactive only, cold-reactive with wide thermal range, and cold-reactive only). Subsequently, an adsorption procedure using ZZAP-treated allogenic RBCs was performed to determine alloantibody type as described [10]. The least-incompatible blood was selected by testing the reactivity of the patient's serum against >10 ABO-compatible units and choosing those which were best matched. All 161 patients were transfused with RBCs that were the least-incompatible and lacked antigen against any detected alloantibodies.
In addition, 100 anemic patients who were negative in both DAT and autologous control panel, but showed positivity in specific panels on the antibody identification test, and were transfused once with 1-5 units of packed RBCs lacking antigen(s) against the detected alloantibodies were classified as the first control group. In addition, 100 anemic patients who showed negative results in both DAT and antibody identification test, and were transfused once with 1-5 units of ABO-compatible packed RBCs were enrolled as the second control group. All patients with evident bleeding or multiple transfusions during follow-up were excluded. This study was approved by the institutional review board of Asan Medical Center, Seoul, Korea.
Both clinical and laboratory findings for each patient as well as history of fever (>37.3℃) or evidence of acute hemolytic transfusion reaction within 24 hours after transfusion were obtained by retrospective review of electronic medical records, and were compared among the three patient groups. These results are represented in Table 1.
We investigated hemoglobin, total bilirubin, and lactate dehydrogenase (LDH) data after RBC transfusion and calculated the changes compared with pre-transfusion levels. To adjust for the effect of transfused RBC volume and body weight of the patient on the transfusion, we applied the concept of "hemoglobin, total bilirubin, and LDH changes after transfusion of defined amount of 10 mL RBCs/kg body weight of each patient." An example of the calculation is provided below:
(A) Absolute volume of transfused RBCs: 800 mL
(B) Hemoglobin changes: 1.6 g/dL (present level-pre-transfusion level)
(C) Body weight of patient: 60 kg
(D) Absolute volume of transfused RBCs/kg body weight of patient: (A)/(C)=13.3 mL/kg
(E) Hemoglobin changes after 10 mL/kg of transfused RBCs: [(B)×10 mL/kg]/(D)=1.20 g/dL
Using this formula, both absolute levels of three items and their changes were enumerated and compared separately among the three patient groups. These results are summarized in Table 2 (hemoglobin), Table 3 (total bilirubin), and Table 4 (LDH). Schematic illustrations representing their changes are shown in Supplemental Data Figure S1.
In this analysis, we focused on the hemoglobin changes at days 1 and 7 after RBC transfusion, and hemoglobin changes were compared among the patient groups categorized by each variable described previously. These results are illustrated in Fig. 1.
In this analysis, we compared the total units and volume of transfused RBCs, transfused RBCs/kg body weight of patient, absolute hemoglobin levels, and the hemoglobin changes at each follow-up day after transfusion of 10 mL RBCs/kg among the three patient groups. These results are summarized in Table 5. Schematic illustrations representing the trends of hemoglobin changes are shown in Supplemental Data Figure S2.
Pearson chi-square tests were used to compare categorical variables. The Mann-Whitney U test and Kruskal-Wallis test were used to compare median (range) value of continuous variables between two groups and more than two groups, respectively. All statistical analyses were performed using SPSS 13.0.1 for Windows (SPSS Inc, Chicago, IL, USA) and P values ≤0.05 were considered statistically significant.
The AIHA patients showed significant female predominance (P=0.022) and were younger (P<0.001) than those with no antibodies; however, the total units and volume of transfused RBCs were not different among the three patient groups. Many patients with autoantibodies had underlying diseases, including hematologic malignancy (20.5%), solid tumors (24.8%), autoimmune disease (8.7%), and infection (5.6%). Approximately a half of the patients with autoantibodies (54.0%) possessed underlying alloantibodies, including anti-E and -c (24.8%), anti-C and -e (4.3%), anti-E (3.1%), and anti-M (3.1%).
AIHA patients received significantly more steroid therapy (P=0.001) and tended towards more frequent immunosuppressive therapy (P=0.081) than those with alloantibodies only. Twenty-five alloantibody patients received steroids only when they were transfused, with underlying diseases that included solid tumor (10 patients), autoimmune disease (four patients), infection (three patients), osteoarthritis (two patients), and others such as chronic renal failure and epidural hemorrhage (six patients). The patients with autoantibodies showed significantly worse anemia than those with alloantibodies only (P<0.001) and those with no antibodies (P<0.001). Among patients with autoantibodies, three patients (1.9%) developed fever (37.9℃, 38.1℃, and 37.7℃ peak temperatures) within 24 hrs of transfusion; however, the patients had pneumonia and showed no evidence of hemolytic transfusion reaction (Table 1).
The patients with autoantibodies showed significantly lower hemoglobin levels than those with alloantibodies only at pre-transfusion and from day 1 to day 4; however, no significant differences were observed from day 5 to day 7, indicating a preferential hemoglobin recovery in patients with autoantibodies at long term follow-up compared with those with alloantibodies only. When compared with the normal control group (no antibodies), autoantibody patients exhibited significantly lower hemoglobin levels consistently.
Nonetheless, the three patient groups exhibited similar hemoglobin changes of 1.40-1.70 g/dL (autoantibodies), 1.20-1.60 g/dL (alloantibodies only), and 1.40-1.55 g/dL (no antibodies) after transfusion of 10 mL RBCs/kg. The differences were not significant in all comparisons throughout the seven-day follow-up period, implying a similar benefit of RBC transfusion among the three patient groups (Table 2).
In addition, three patient groups also showed similar total bilirubin changes after transfusion of 10 mL RBCs/kg. The differences were not significant throughout the follow-up period. Notably, patients in the autoantibody group showed no or negative total bilirubin changes consecutively after two days of RBCs transfusion, indicating the absence of hemolysis risk (Table 3). The comparison of LDH changes also showed identical results (Table 4).
Associations between autoantibody type, DAT specificity, DAT strength, and steroid therapy status with hemoglobin changes at day 1 and day 7 after transfusion were not evident. However, patients with more severe pre-transfusion anemia showed significantly greater hemoglobin changes at day 7 after than those with mild anemia (P<0.001). This result may suggest an increased benefit of transfusion in patients with more severe autoimmune anemia compared with those with milder forms (Fig. 1).
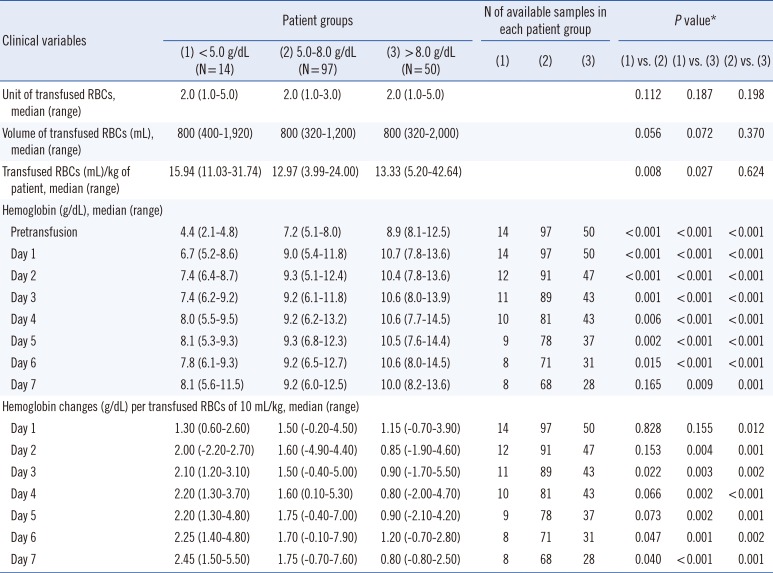
When the body weight of patient was normalized, patients with initial hemoglobin levels <5 g/dL received significantly higher amount of transfused RBCs per body weight of patient than those with initial levels 5-8 g/dL or >8 g/dL, denoting a higher absolute volume of transfused RBCs in patients with initial hemoglobin levels <5 g/dL. Furthermore, patients with initial hemoglobin levels <5 g/dL displayed significantly lower hemoglobin levels than those at 5-8 g/dL from day 1 to day 6; however, the hemoglobin levels at post-transfusion day seven were not significantly different between two subgroups, indicating a more satisfactory hemoglobin recovery in patients with severe anemia at long term follow-up than those with moderate anemia. In contrast, those with initial hemoglobin levels <5 g/dL showed significantly lower hemoglobin levels consistently during all follow-up days compared with the patients with initial levels >8 g/dL.
In addition, patients with initial hemoglobin levels <<5 g/dL revealed more hemoglobin changes from day two compared with other patient groups. The differences were statistically significant at day 3, day 6, and day 7 compared with the patients with initial hemoglobin levels 5-8 g/dL, and were consistently significant from days 2-7 compared with those with initial levels >8 g/dL; it suggests that patients with severe anemia consistently exhibit a significantly greater degree of transfusion benefit compared with those with mild-moderate anemia (Table 5).
RBC transfusion to patients with autoantibodies was classically regarded to be therapeutically non-beneficial owing to the difficulties of finding compatible blood donor and increased risk of transfusion reactions [4, 11]. A study on Jehovah's Witness patients with hemoglobin <5 g/dL showed inconsistent mortality rates related to severe anemia with transfusion refusal, demonstrating that a portion of severely anemic patients can survive without transfusion [12]. In contrast, recent reports propose that it may be more beneficial to transfuse incompatible RBCs than to refrain from treatment, especially in patients with cardiovascular disease or severely symptomatic patients in urgent need of additional oxygen-carrying capacity [1, 2, 4, 11]. To date, an intensive clinical study focusing on the efficacy of transfusion in AIHA patients has not been performed.
In the present study, many AIHA patients had underlying diseases including hematologic malignancy, solid tumor, autoimmune disease, and infection. This result is consistent with previous studies [1, 2] and supports recent hypothesis that autoantibodies found in AIHA may be a result of immune reactions in response to foreign pathogen. Significantly, our study revealed that although initial hemoglobin levels prior to transfusion were lower in patients with autoantibodies than those with alloantibodies only, patients with autoantibodies recovered preferentially at long-term follow-up compared with those with alloantibodies only. We also found that transfusion efficacy in AIHA patients is not inferior if appropriately transfused with the least-incompatible RBCs and found no evidence of acute hemolytic transfusion reaction within 24 hr of transfusion. Furthermore, transfusion-induced changes in both total bilirubin and LDH were not significantly different among three patient groups, with the AIHA patients showing an absence or negative total bilirubin and LDH changes consistently after post-transfusion day 2. Altogether, these results support the safety and efficacy of RBC transfusion in AIHA patients.
Theoretically, the strength of DAT positivity in AIHA patients would be positively correlated with the degree of RBC destruction. Previous studies reported that 86% of patients with DAT positivity who experienced hemolysis had strength of ≥2+ [5], and that severe hemolysis is associated with stronger DAT positivity [11]. However, it has also been shown that the strength of the antiglobulin reaction and RBCs destruction rates correlate poorly [13, 14]. Therefore, the effect of DAT strength on hemolysis is currently under debate [15]. Our study found no significant correlation between DAT strength and hemoglobin changes, and supports previous studies describing no significant influence on transfusion efficacy by either autoantibody type or DAT specificity [13, 14]. A possible hypothesis for this phenomenon is that RBC destruction rates are partially related to IgG autoantibody subclasses, as both affinity to the macrophage Fc receptor and their complement-fixing activity differ among antibody subclasses [1].
Interestingly, although the absolute volumes of transfused RBCs were significantly greater in patients with severe anemia, the therapeutic effect of the transfusion was significantly greater for seven consecutive days after transfusion in the patients with severe anemia compared with those with mild-moderate anemia. This supports previous guidelines that considered transfusion in symptomatic patients with RBC autoantibodies [1, 2, 4, 8, 11]. Additionally, we found no significant correlation between steroid therapy status and transfusion efficacy. This result is opposite to previous beliefs suggesting steroids as a therapeutic mainstay in AIHA. However, as we did not include the patients receiving steroid therapy alone as a comparable patient group, our result should be interpreted with caution and a further study should be performed for confirmation.
Our study has a limitation in the assessment of hemolysis risk, since only total bilirubin and LDH were used as hemolysis markers, rather than serum haptoglobin and plasma hemoglobin levels. This limitation was from the retrospective study design, since analysis of these markers is not routinely performed in the clinic. Thus, the results presented here should be interpreted with caution until a further comprehensive study is completed.
In conclusion, the transfusion of the least-incompatible RBCs to the patients with autoantibodies yielded similar increases in hemoglobin levels without an associated risk of hemolysis compared with those patients with alloantibodies only or without antibodies. In addition, autoantibody type, DAT specificity, DAT strength, and steroid therapy status had no influence on transfusion efficacy. This suggests that RBC transfusion is expected to be an effective and safe method of therapy in AIHA patients, especially in those with severe anemia.
References
1. Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev. 2008; 22:17–31. PMID: 17904259.

2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010; 24:195–210. PMID: 20656187.

4. Reardon JE, Marques MB. Laboratory evaluation and transfusion support of patients with autoimmune hemolytic anemia. Am J Clin Pathol. 2006; 125(S):S71–S77. PMID: 16830958.

5. Wheeler CA, Calhoun L, Blackall DP. Warm reactive autoantibodies: clinical and serologic correlations. Am J Clin Pathol. 2004; 122:680–685. PMID: 15491963.
6. Roback JD, Combs MR, editors. Technical manual. 16th ed. Bethesda: American Association of Blood Banks;2008. p. 511.
7. Hillyer CD, Silberstein LE, editors. Blood banking and transfusion medicine. 2nd ed. Philadelphia: Elsevier;2007. p. 560–561.
8. Salama A, Berghöfer H, Mueller-Eckhardt C. Red blood cell transfusion in warm-type autoimmune haemolytic anaemia. Lancet. 1992; 340:1515–1517. PMID: 1361607.

9. Petz LD. A physician's guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol. 2004; 124:712–716. PMID: 15009058.

10. Leger RM, Garratty G. Evaluation of methods for detecting alloantibodies underlying warm autoantibodies. Transfusion. 1999; 39:11–16. PMID: 9920161.

11. King KE, Ness PM. Treatment of autoimmune hemolytic anemia. Semin Hematol. 2005; 42:131–136. PMID: 16041662.

12. Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah's Witnesses. Transfusion. 1994; 34:396–401. PMID: 8191563.

13. Wikman A, Axdorph U, Gryfelt G, Gustafsson L, Björkholm M, Lundahl J. Characterization of red cell autoantibodies in consecutive DAT-positive patients with relation to in vivo haemolysis. Ann Hematol. 2005; 84:150–158. PMID: 15551098.

14. Garratty G, Nance SJ. Correlation between in vivo hemolysis and the amount of red cell-bound IgG measured by flow cytometry. Transfusion. 1990; 30:617–621. PMID: 2402775.

15. Garratty G. The James Blundell Award Lecture 2007: do we really understand immune red cell destruction? Transfus Med. 2008; 18:321–334. PMID: 19140815.

Supplementary Materials
Supplemental Data Figure S1
Comparison of (A) hemoglobin, (B) total bilirubin, and (C) lactate dehydrogenase changes (medians) during a seven-day follow-up period after transfusion of 10 mL RBCs/kg body weight among patients with autoantibodies±alloantibodies, patients with alloantibodies only, and patients without antibodies.
Supplemental Data Figure S2
Comparison of hemoglobin changes (medians) during a seven-day follow-up period after transfusion of 10 mL RBCs/kg body weight in patients with autoantibodies with respect to their pre-transfusion hemoglobin levels.
Fig. 1
Comparison of hemoglobin changes (medians) at days 1 and 7 after transfusion of 10 mL RBCs/kg in the patients with autoantibodies with respect to (A) autoantibody type, (B) DAT specificity, (C) DAT strength in IgG, (D) DAT strength in C3d, (E) initial hemoglobin level, and (F) steroid therapy status.
*P values were obtained from the Kruskal-Wallis test and †the Mann-Whitney U test.
Abbreviations: RBC, red blood cell; DAT, direct antiglobulin test.
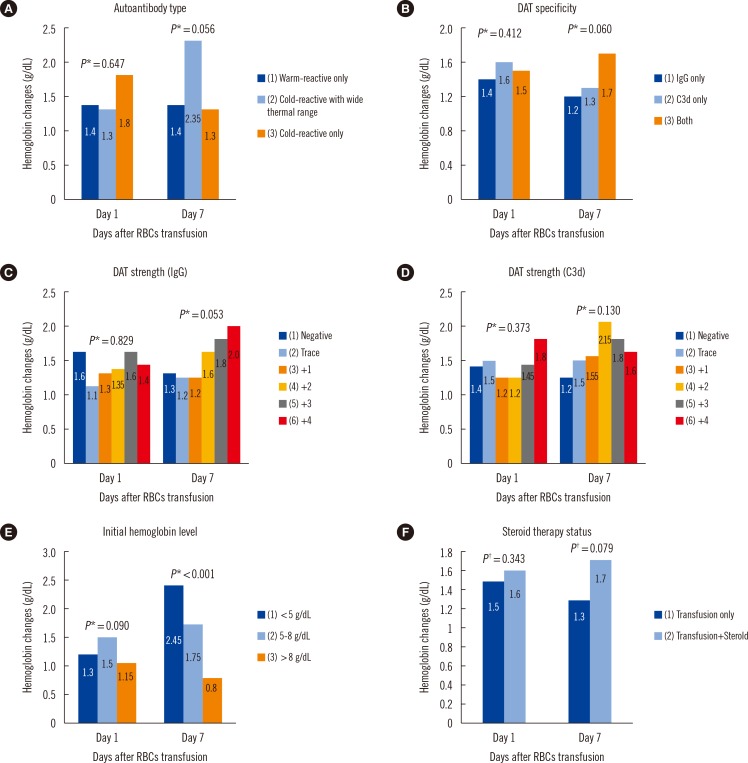
Table 1
Comparison of clinical and laboratory findings of total 361 patients among three patient groups categorized by detected type of antibodies
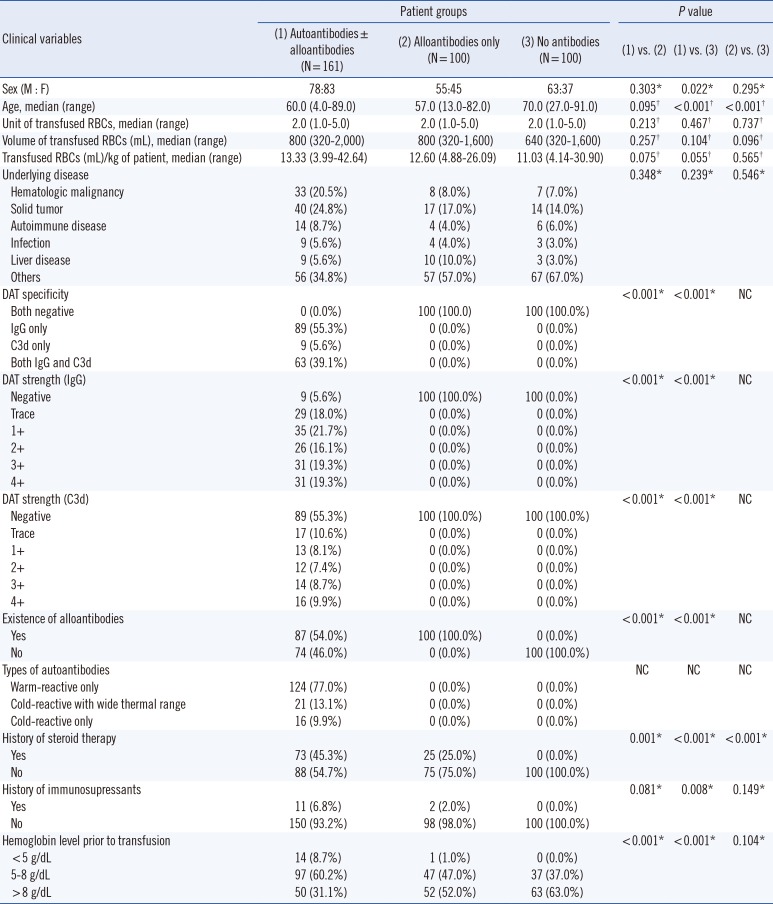
Table 2
Comparison of hemoglobin levels and their changes at each follow-up day after transfusion among three patient groups categorized by detected type of antibodies
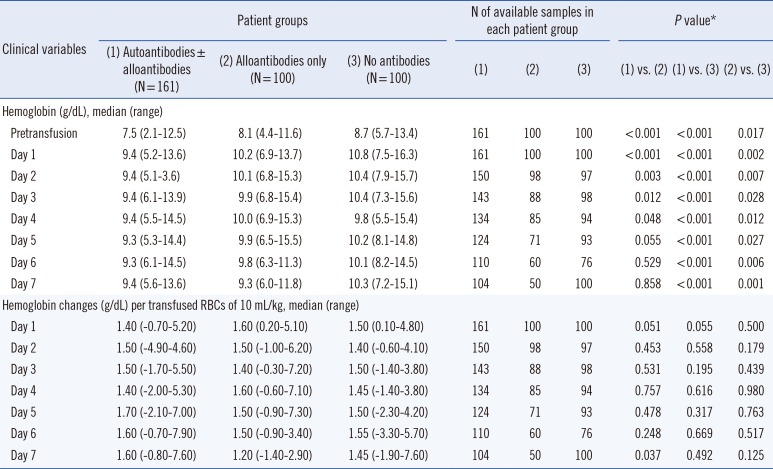
Table 3
Comparison of serum total bilirubin levels and their changes at each follow-up day after transfusion among three patient groups categorized by detected type of antibodies
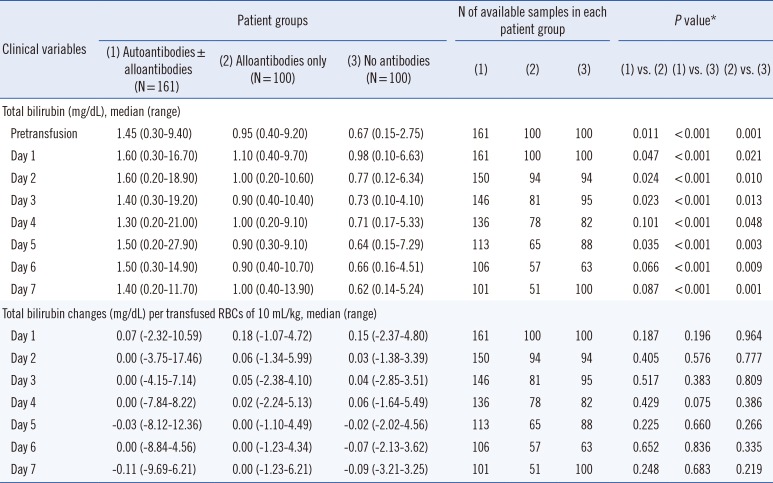
Table 4
Comparison of serum lactate dehydrogenase levels and their changes at each follow-up day after transfusion among three patient groups categorized by detected type of antibodies
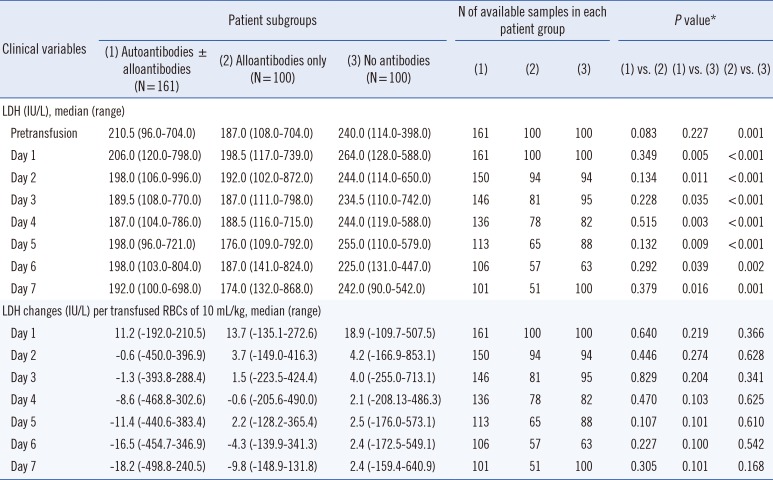
Table 5
Comparison of hemoglobin levels and their changes at each follow-up day after transfusion in the patients with autoantibodies with respect to their initial hemoglobin levels prior to RBCs transfusion




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download