Abstract
Background
Streptococcus pneumoniae causes pneumonia, sepsis, and meningitis. This study aimed to investigate the clinical characteristics of mucoid and non-mucoid isolates of S. pneumoniae, and to explore the relationship between the isolate phenotypes and their antibiotic susceptibility.
Methods
Clinical isolates from 3,453 non-repetitive S. pneumoniae (189 mucoid and 3,264 non-mucoid) infections obtained between January 2008 and December 2012 from outpatients at the Kimitsu-Central Hospital were evaluated.
Results
Compared to the non-mucoid isolates, the mucoid phenotypes were more susceptible to certain antibiotics such as erythromycin, clarithromycin, and tetracycline as opposed to clindamycin, chloramphenicol, and rifampicin. The mucoid phenotype was isolated more frequently from schoolchildren, adults, and elderly adults in a variety of clinical sites, including otorrhea, genitalia, pus, and eye discharge than the non-mucoid phenotype. This suggested that mucoid isolates are more likely to be involved than non-mucoid isolates in various local infections. Systemic infection, which indicates invasiveness, was not associated with the mucoid or non-mucoid phenotype.
Conclusions
The results of this study suggest that mucoid isolates tend to have higher susceptibility than non-mucoid isolates to antibiotics. To the best of our knowledge, mucoid and non-mucoid S. pneumoniae isolates considerably differ in terms of clinical isolation site and age-specific prevalence.
Streptococcus pneumoniae is an important pathogen that causes invasive and non-invasive pneumococcal diseases (e.g., meningitis, sepsis, pneumonia, and otitis media) in individuals of all age groups [1]. In various countries including Japan, pneumococcal conjugate vaccines are routinely used to protect infants, children, and elderly adults from pneumococcal diseases; severe pneumococcal pneumonia and meningitis are still associated with high mortality rates [2]. Furthermore, antimicrobial resistance in S. pneumoniae has been observed globally since 1980s [3]. In particular, the increasing resistance of S. pneumoniae strains to widely used anti-pneumococcal drugs, including ß-lactams and macrolides, has become a serious clinical concern in both developing and developed countries, including Japan [4, 5].
Colonies of S. pneumoniae isolates show various morphological features, such as the presence of a central depression or a mucoid appearance [6]. The mucoid phenotype has also been observed in other pathogenic bacteria, including Pseudomonas aeruginosa [7]. A previous study showed that the minimal inhibitory concentration (MIC) of various antibiotics in P. aeruginosa with the mucoid phenotype is lower than that in non-mucoid strains [8]. However, the relationship between antibiotic susceptibility and the mucoid phenotype in S. pneumoniae remains unclear. Therefore, in the present study, the relationship between the mucoid phenotype and the antibiotic susceptibility patterns of S. pneumoniae isolates from outpatients with a variety of infections was examined.
In this study, 3,453 non-repetitive S. pneumoniae (189 mucoid and 3,264 non-mucoid) clinical isolates were evaluated; they were obtained between January 2008 and December 2012 from outpatients at the Kimitsu-Central Hospital who were suspected to have infectious diseases. Strains isolated from hospitalized patients within 48 hr were defined as outpatient-derived strains.
To elucidate the age-specific prevalence of different phenotypes, the patients were assigned to one of four different age groups. Patients aged 0-5 yr were assigned to the infant and preschool group; those aged 6-11 yr were assigned to the schoolchildren group; those aged 12-64 yr were assigned to the adult group, and those aged ≥65 yr were assigned to the elderly adult group.
All 3,453 strains were identified by using an optochin disk (Eiken Chemical Co. Ltd., Tokyo, Japan) and Slidex pneumo-Kit (Sismex bioMerieux, Tokyo, Japan). Mucoid strains were visually identified on the basis of colony morphology described in the Manual of Clinical Microbiology, 10th edition [9]. Susceptibility to penicillin G (meningitis and nonmeningitis), amoxicillin-clavulanate, cefotaxime (meningitis and nonmeningitis), ceftriaxone (meningitis and nonmeningitis), cefepime (meningitis and nonmeningitis), meropenem, erythromycin, clarithromycin, clindamycin, tetracycline, chloramphenicol, vancomycin, rifampicin, and trimethoprim-sulfamethoxazole was tested by using MicroFAST 5J panels (Siemens Healthcare Diagnostics, Tokyo, Japan). Only susceptibility to ceftriaxone was evaluated in 57 mucoid and 1,226 non-mucoid samples isolated after May 2011. The results were interpreted according to the Clinical Laboratory Standards Institute document M100-S24 [10]. Oral penicillin V is an unapproved antimicrobial agent in Japan; therefore, it was not evaluated in this study. The MicroFAST 5J panels were inoculated and read according to the manufacturer's recommendations. The inoculated panels were then incubated at 35℃ in ambient air for 20-24 hr prior to visual determination of the MIC. Quality control for MIC determination was performed by using the reference strain S. pneumoniae ATCC 49619.
Data were analyzed by using Microsoft Excel for Mac 2011. Categorical variables were compared by using either chi-squared test or Fisher's exact test, as appropriate. Two-tailed P<0.05 was considered statistically significant. Statistical significance was assessed in comparison between the infant and preschool group and each age group for antibiotic resistance rate with either chi-squared test or Fisher's exact test. When the infant and preschool group showed significance to all each age group, we defined this was significant.
The MIC50 of penicillin G (nonmeningitis), cefotaxime (nonmeningitis), ceftriaxone (nonmeningitis), and meropenem in non-mucoid isolates was higher than that in mucoid isolates (Table 1). Similarly, the MIC90 of amoxicillin-clavulanate, cefepime (nonmeningitis), and trimethoprim-sulfamethoxazole in non-mucoid isolates was higher than that in mucoid isolates. Since only non-mucoid isolates were detected from patients with meningitis, we compared mucoid isolates and non-mucoid isolates from patients with meningitis with regard to their susceptibility to penicillin G, cefotaxime, ceftriaxone, and cefepime (Table 1). Additionally, non-mucoid isolates from patients with meningitis before 2010 were not tested for ceftriaxone susceptibility (meningitis). The MIC50 and MIC90 of erythromycin, clarithromycin, tetracycline, vancomycin, levofloxacin, and rifampicin were comparable for mucoid and non-mucoid isolates, although clarithromycin, tetracycline, and vancomycin are not routinely used in the clinical setting. The rate of resistance and MIC of protein synthesis-inhibiting antibiotics, including erythromycin, clarithromycin, clindamycin, tetracycline, and chloramphenicol, were found to be high for both. Moreover, the rate of resistance to clarithromycin, tetracycline, and trimethoprim-sulfamethoxazole was higher in non-mucoid isolates than in mucoid isolates (P<0.01); the rate of resistance to clindamycin, chloramphenicol, and rifampicin was higher in mucoid isolates than in non-mucoid isolates (P<0.01) (Table 1). However, the rate of resistance to β-lactam antibiotics, including penicillin G, amoxicillin-clavulanate, cefotaxime, ceftriaxone, cefepime, meropenem, and vancomycin, did not differ significantly between the two phenotypes (Table 1). All mucoid and non-mucoid strains isolated from meningitis samples were susceptible to both penicillin G and cefotaxime, which have two cut-off values in meningitis and non-meningitis samples.
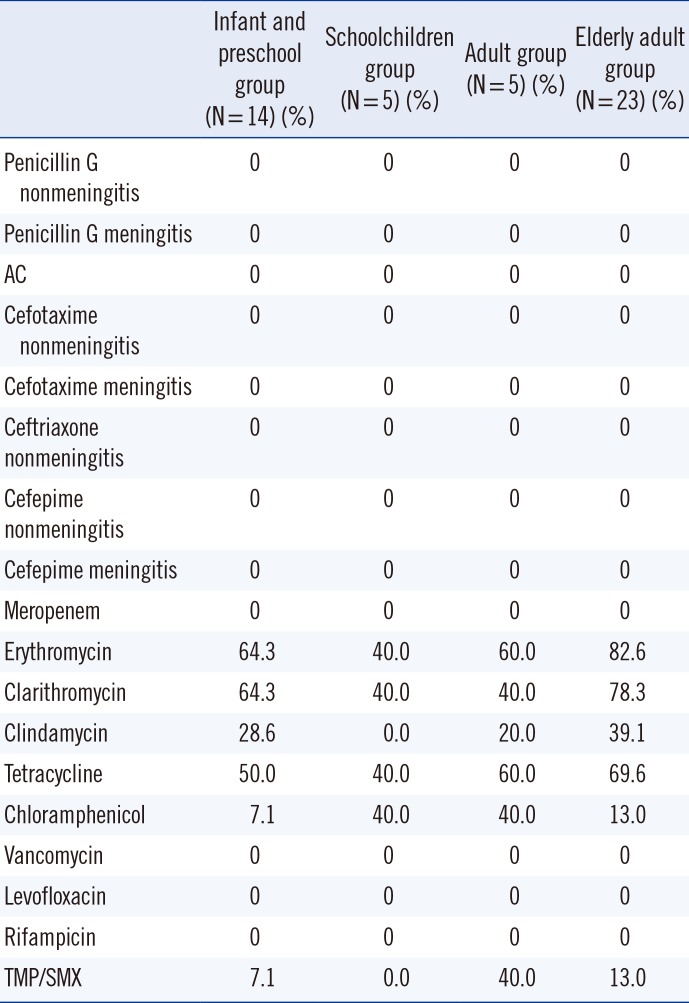
Among the schoolchildren, adult, and elderly adult groups, the frequency of mucoid isolates was significantly higher than that of non-mucoid isolates (P<0.01) (Table 2). Non-mucoid isolates were more resistant to erythromycin, clarithromycin, clindamycin, and tetracycline in the infant and preschool group than in the schoolchildren, adult, and elderly adult groups in all samples (P<0.05) (Table 3). The results for non-mucoid isolates were similar for all respiratory tract samples (Table 4). The invasiveness and antibiotic resistance rate of non-mucoid isolates did not differ significantly among age groups for all antibiotics (Table 5). The antibiotic resistance rate did not differ significantly among mucoid isolates, too. In contrast, the frequency of mucoid isolates obtained from otorrhea samples, genital swabs, ecthyma and abscess exudate, and eye discharge was significantly higher than that of non-mucoid isolates (P<0.05) (Table 2).
Mucoid colonies of S. pneumoniae generate large amounts of capsular polysaccharides [9]. The capsule polysaccharide is the major virulence determinant of S. pneumoniae [11], particularly in hosts lacking type-specific antibodies of sufficient quantity or avidity [12]. Moreover, previous studies demonstrated that the mucoid phenotype, which is characterized by the presence of hyaluronic acid capsular polysaccharide in Streptococcus pyogenes, is a key virulence determinant associated with severe S. pyogenes infections [13, 14]. The mucoid phenotype of S. pneumoniae may also show higher pathogenicity than the non-mucoid phenotype; therefore, it is important to characterize S. pneumoniae, especially the mucoid phenotype, with regard to antibiotic susceptibility.
Previous studies demonstrated that P. aeruginosa isolates with mucoid phenotype are more susceptible than non-mucoid isolates to multiple antibiotics such as β-lactams and fluoroquinolones [8, 15]. Although the reason for antibiotic resistance was not identified in the present study, Ciofu et al. [8] provided a possible explanation for the antibiotic susceptibility of mucoid-overproducing P. aeruginosa; they proposed that non-mucoid resistant isolates co-existing with mucoid strains in biofilms may play a protective role. This explanation could be also applied to S. pneumoniae. Although similar findings have been reported in P. aeruginosa [8], to the best of our knowledge, this is the first report showing differences in antibiotic susceptibility patterns of mucoid and non-mucoid S. pneumoniae isolates.
S. pneumoniae infections are more common in infants, children, and elderly adults than in adults. In this study, it was observed that mucoid isolate infections were not age-dependent, whereas non-mucoid isolates were more frequent in infants and preschool children. Pastor et al. [16] reported an age-specific S. pneumoniae prevalence of approximately 35-45% in infants and preschool children, and 20% in elderly adults. In the present study, the prevalence of mucoid isolates in the elderly adult group was approximately 10% higher than that reported by Pastor et al.[16], whereas the prevalence of non-mucoid isolates was found to be approximately 35% higher in the infant and preschool group and 10% lower in the elderly adult group. Mason et al. [17] noted that younger age is a risk factor for infection with a penicillin-resistant isolate. The present study did not evaluate penicillin-non-susceptible isolates; rather, it showed resistance to protein synthesis-inhibiting antibiotics. We observed that younger age is associated with isolation of strains not susceptible to protein synthesis-inhibiting antibiotics. Regarding the similar results between respiratory tract samples and overall set of all samples, it should be noted that of the 3,264 isolates, 79% (3,161) were derived from respiratory tract samples.
Most non-mucoid isolates were obtained from patients with respiratory infections, which may suggest that mucoid isolates are more variable in terms of clinical infection site than non-mucoid phenotypes isolated from the respiratory system. However, systemic infection, which indicates invasiveness, was not associated with either of the mucoid and non-mucoid phenotypes.
In conclusion, to the best of our knowledge, this study is that mucoid and non-mucoid S. pneumoniae isolates differ significantly in terms of clinical site of isolation and age-specific prevalence. In particular, these results suggest that mucoid isolates tend to have greater antibiotics susceptibility than non-mucoid isolates. However, limited studies on the region-specific epidemiology of mucoid and non-mucoid isolates have been reported. Furthermore, this study was not designed to collect information regarding serotype distributions. Therefore, further studies to confirm the present findings are warranted.
References
1. Fine MJ, Orloff JJ, Arisumi D, Fang GD, Arena VC, Hanusa BH, et al. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med. 1990; 88(5N):1N–8N. PMID: 2294759.
2. Chiba N, Morozumi M, Shouji M, Wajima T, Iwata S, Sunakawa K, et al. Rapid decrease of 7-valent conjugate vaccine coverage for invasive pneumococcal diseases in pediatric patients in Japan. Microb Drug Resist. 2013; 19:308–315. PMID: 23480525.

3. Adam D. Global antibiotic resistance in Streptococcus pneumoniae. J Antimicrob Chemother. 2002; 50(Suppl):1–5. PMID: 12077153.

4. Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004; 48:2101–2107. PMID: 15155207.
5. Baquero F. Pneumococcal resistance to beta-lactam antibiotics: a global geographic overview. Microb Drug Resist. 1995; 1:115–120. PMID: 9158743.
6. Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. Accuracy of phenotypic methods for identification of Streptococcus pneumoniae isolates included in surveillance programs. J Clin Microbiol. 2008; 46:2184–2188. PMID: 18495854.
7. Speert DP, Farmer SW, Campbell ME, Musser JM, Selander RK, Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990; 28:188–194. PMID: 2107198.

8. Ciofu O, Fussing V, Bagge N, Koch C, Høiby N. Characterization of paired mucoid/non-mucoid Pseudomonas aeruginosa isolates from Danish cystic fibrosis patients: antibiotic resistance, beta-lactamase activity and RiboPrinting. J Antimicrob Chemother. 2001; 48:391–396. PMID: 11533004.

9. Versalovic J, Carroll KC, et al. Manual of clinical microbiology. 10th ed. Washington DC: American Society for Microbiology;2011. p. 331–349.
10. Clinical and Laboratory Standards Institute. Reference method for broth dilution antimicrobial susceptibility testing. Approved standard. M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute;2014.
11. Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995; 33:2759–2762. PMID: 8567920.
12. Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998; 177:368–377. PMID: 9466523.
13. Gryllos I, Tran-Winkler HJ, Cheng MF, Chung H, Bolcome R 3rd, Lu W, et al. Induction of group A Streptococcus virulence by a human antimicobial peptide. Proc Natl Acad Sci U S A. 2008; 105:16755–16760. PMID: 18936485.
14. Kakuta R, Yano H, Hidaka H, Miyazaki H, Irimada M, Oda K, et al. Severe acute otitis media caused by mucoid streptococcus pyogenes in a previously healthy adult. Tohoku J Exp Med. 2014; 232:301–304. PMID: 24727832.
15. Shawar RM, MacLeod DL, Garber RL, Burns JL, Stapp JR, Clausen CR, et al. Activities of tobramycin and six other antibiotics against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 1999; 43:2877–2880. PMID: 10582875.
16. Pastor P, Medley F, Murphy TV. Invasive pneumococcal disease in Dallas County, Texas: results from population-based surveillance in 1995. Clin Infect Dis. 1998; 26:590–595. PMID: 9524828.

17. Mason EO Jr, Lamberth LB, Kershaw NL, Prosser BL, Zoe A, Ambrose PG. Streptococcus pneumoniae in the USA: in vitro susceptibility and pharmacodynamic analysis. J Antimicrob Chemother. 2000; 45:623–631. PMID: 10797084.

Table 1
MIC50, MIC90, and range of various antibiotics for Streptococcus pneumoniae
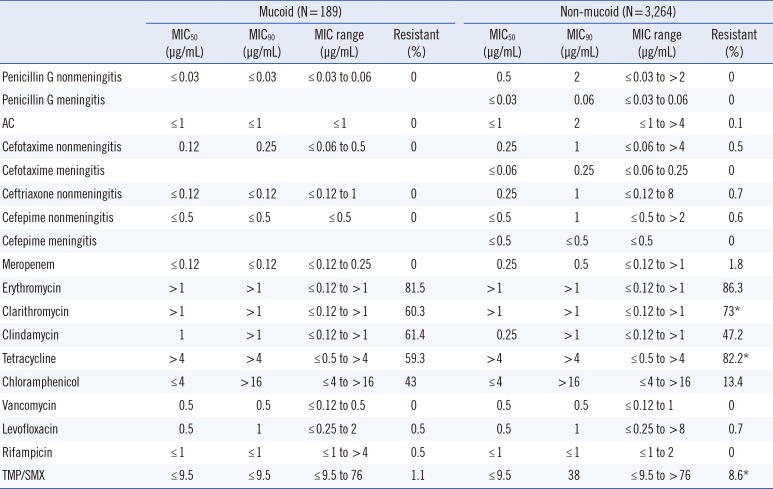
Table 2
Patient age at the time of detection and tissue source for Streptococcus pneumoniae isolated from 189 patients infected with mucoid strains and 3,264 patients infected with non-mucoid strains
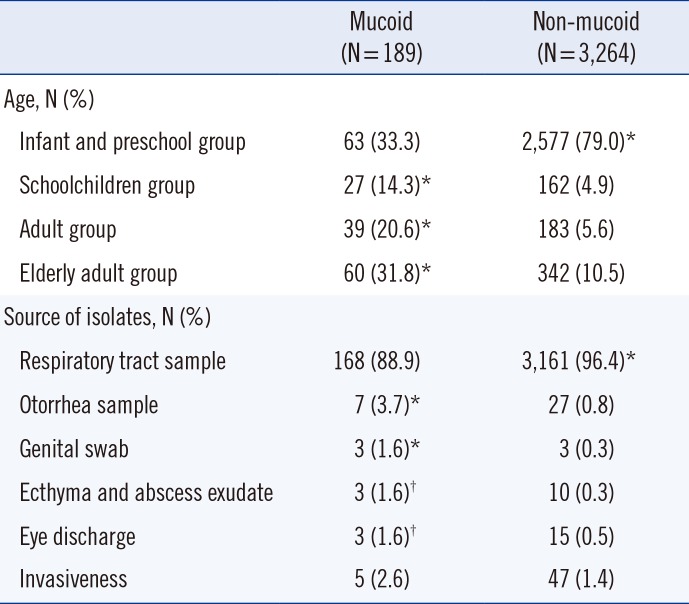
Table 3
Antibiotic resistance rate (%) of non-mucoid strains isolated from outpatients according to their age group, including those with meningitis and nonmeningitis, in all samples
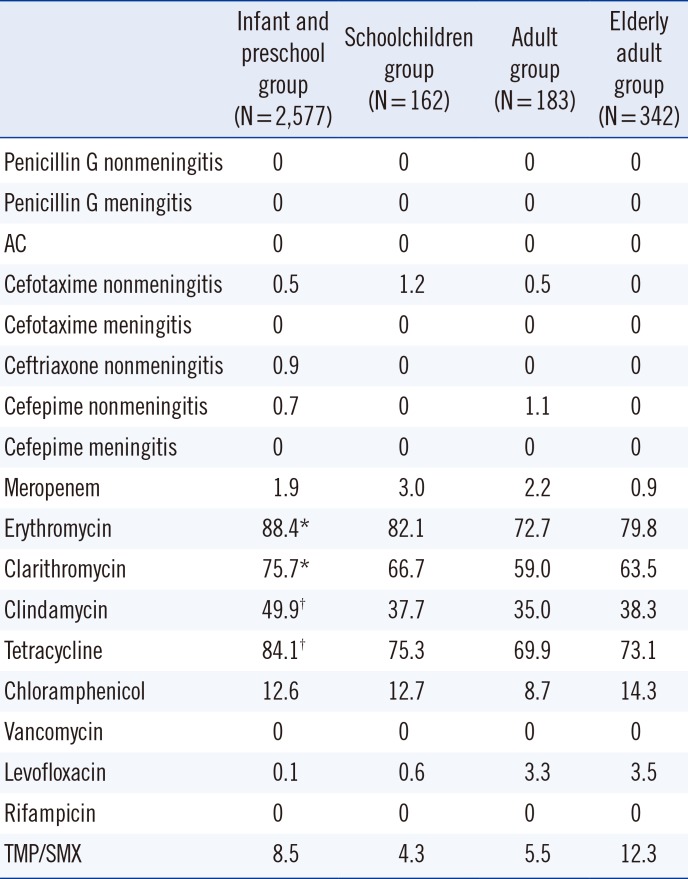
Table 4
Antibiotic resistance rate (%) of non-mucoid strains isolated from outpatients according to their age group, for respiratory tract samples
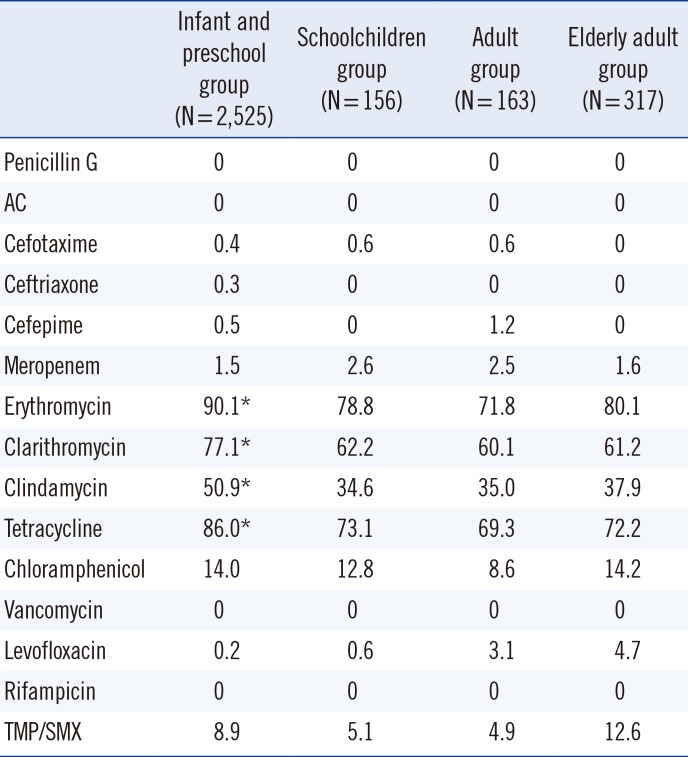
Table 5
Antibiotic resistance rate (%) of non-mucoid strains isolated from outpatients according to their age group, including those with meningitis and nonmeningitis, with regard to invasiveness




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download