Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia with a vancomycin minimum inhibitory concentration (MIC) of 2 µg/mL presents a high rate of therapeutic failure in response to vancomycin. In addition, polymorphism in accessory gene regulator (agr) is associated with vancomycin therapeutic effects. The association between agr polymorphism and vancomycin MICs was investigated in MRSA isolates.
Methods
Agr group-specific PCR was conducted on 118 MRSA bloodstream isolates. Vancomycin susceptibility tests were conducted, while E-test GRD (bioMérieux SA, France) was used to detect heterogeneous vancomycin-intermediate S. aureus (hVISA).
Results
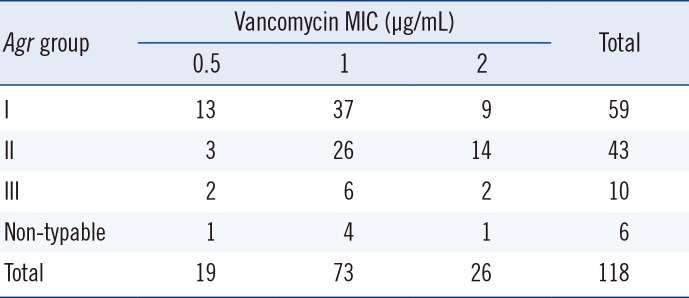
Of the 118 MRSA isolates, 59 (50.0%), 43 (36.4%), and 10 (8.5%) isolates belonged to agr group I, II, and III, respectively. Six isolates could not be classified. Twenty-six, 73, and 19 isolates presented a vancomycin MIC of 2, 1, and 0.5 µg/mL, respectively. Nine (34.6%), 14 (53.8%), and 2 (7.7%) isolates with MICs of 2 µg/mL belonged to agr group I, II, and III, respectively. Thirty-seven (50.6%), 26 (35.6%), and 6 (8.2%) isolates with MICs of 1 µg/mL belonged to agr group I, II, and III, respectively. Thirteen (68.4%), 3 (15.8%), and 2 (10.5%) isolates with MICs of 0.5 µg/mL belonged to agr group I, II, and III, respectively. The agr group II presented more isolates with MIC of 2 µg/mL (32.6%) than the agr non-group II (16%). Four isolates tested positive for hVISA. Three of them belonged to agr group II.
In Staphylococcus aureus vancomycin susceptibility test, a minimum inhibitory concentration (MIC) of 16 µg/mL or more is categorized as resistant, 4-8 µg/mL as intermediate, and 2 µg/mL or less as susceptible according to CLSI criteria [1]. Glycopeptides such as vancomycin and teicoplanin are used as primary therapeutic options for methicillin-resistant S. aureus (MRSA) infection [2, 3]. Although MRSA infection with a vancomycin MIC of 2 µg/mL is categorized as susceptible according to the criteria of antibiotic susceptibility test, treatment using glycopeptides presents a high rate of failure [4]. Furthermore, MRSA with a vancomycin MIC of 2 µg/mL and MRSA with a vancomycin MIC of 1 µg/mL need to be distinguished [4, 5]. Even though vancomycin MICs are mainly determined by using automated equipment, differences in measurements may be observed when using theautomated antibiotic susceptibility test versus using CLSI reference method [6].
Accessory gene regulator (agr) operon participates in the regulation of virulence factors of S. aureus. S. aureus is divided into four agr groups based on its amino acid sequence polymorphism [7, 8]. It has been reported that agr polymorphism is related to vancomycin intermediate S. aureus (VISA) [9] and to failure of glycopeptide treatment for MRSA infections [8, 10].
VISA that shows heteroresistance to vancomycin (hVISA) has been increasing in frequency, since it was first reported in Japan in 1997 [11, 12]. The automated antibiotic susceptibility test used in most clinical microbiology laboratories can not detect hVISA [12] and vancomycin MICs higher than 1 µg/mL are related to hVISA [11, 13], thereby, necessitating additional tests for the detection of hVISA.
In MRSA infections, vancomycin MIC of 2 µg/mL, agr group II polymorphism, and hVISA are factors that may be related to treatment failure. This study used MRSA bloodstream isolates to compare vancomycin MICs obtained by CLSI broth microdilution (BMD) to those obtained with an automated susceptibility test and to determine the association between agr polymorphism and vancomycin MIC of 2 µg/mL. Further more, the distribution of hVISA was investigated.
A total of 118 MRSA strains isolated from blood cultures between September 2012 and August 2013 were used in this study. Thirty-six strains were isolated from blood cultures in two teaching hospitals in Seoul and 82 strains were isolated from blood cultures in a teaching hospital in Gyeonggi province in Korea. The isolates were stored at -70℃ and then cultured on blood agar plates. The study was exempt from review by institution review board (IRB) of Hallym University Sacred Heart Hospital.
S. aureus was identified by using MicroScan Pos Combo 28 Panel (Siemens, West Sacramento, CA, USA) and conventional methods such as coagulase test, mannitol fermentation, and DNAse test. The methicillin resistance was determined by resistance to cefoxitin and by PCR to detect the mecA gene [14].
According to the manufacturer's instructions, antibiotic susceptibility test was conducted by using MicroScanPos Combo 28 Panel. Using the BMD method suggested by CLSI [1], MICs were measured for vancomycin concentrations of 0.25-16 µg/mL and were compared with those measured by the MicroScan panel. The rates of vancomycin MIC of 2 µg/mL determined by using the MicroScan panel and those determined by using BMD were compared.
Multiplex PCR of agr group I to IV was conducted on MRSA isolates, as previously described [15].
Using E-test GRD (bioMérieux SA, Marcy I'Etoile, France) and Mueller-Hinton agar with 5% sheep blood, hVISA screening was conducted according to the manufacturer's instructions [16].
Agr group-specific PCR showed a total of 118 isolates, 59 (50.0%) isolates belonged to agr group I, 43 (36.4%) isolates belonged to agr group II, and 10 (8.5%) isolates belonged to agr group III. Agr group IV was not detected. Six isolates tested negative for all agr groups. Of the 36 isolates from two hospitals in Seoul, 17 (47.2%) isolates belonged to agr group I, 14 (38.9%) isolates belonged to agr group II, and four (11.1%) isolates belonged to agr group III. Of the 82 isolates from a hospital in Gyeonggi province, 42 (51.2%) isolates belonged to agr group I, 29 (35.4%) isolates belonged to agr group II, and six (7.3%) isolates belonged to agr group III.
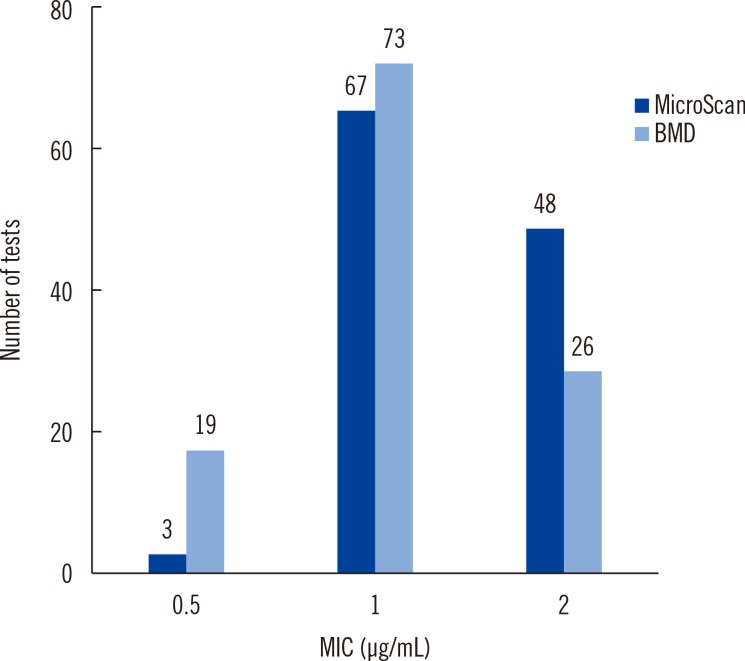
Vancomycin susceptibility results from both BMD and MicroScan panel showed that the MICs of all 118 isolates were 2 µg/mL or less, corresponding to the susceptible category. Vancomycin susceptibility test using BMD showed that the MIC of 26 isolates was 2 µg/mL and that of 92 isolates was 1 µg/mL or less. On the other hand, vancomycin susceptibility test using the MicroScan panel showed that the MIC of 48 isolates was 2 µg/mL and that of 70 isolates was 1 µg/mL or less (Fig. 1). The number of isolates with MIC of 2 µg/mL measured by vancomycin susceptibility test using the MicroScan panel was significantly higher than that determined using BMD (P=0.002).
Of the 26 isolates with vancomycin MIC of 2 µg/mL measured by BMD, nine (34.6%) isolates belonged to agr group I, 14 (53.8%) isolates belonged to agr group II, and two (7.7%) isolates belonged to agr group III. Of the 73 isolates with vancomycin MIC of 1 µg/mL measured by BMD, 37 (50.6%) isolates belonged to agr group I, 26 (35.6%) isolates belonged to agr group II, and six (8.2%) isolates belonged to agr group III. Of the 19 isolates with vancomycin MIC of 0.5 µg/mL measured by BMD, 13 (68.4%) isolates belonged to agr group I, three (15.8%) isolates belonged to agr group II, and two (10.5%) isolates belonged to agr group III. The percentage of isolates with vancomycin MIC of 2 µg/mL measured by BMD was 15.3% (9/59) in agr group I, 32.6% in agr group II, and 20.0% (2/10) in agr group III (Table 1). The percentage of isolates with vancomycin MIC of 2 µg/mL in agr group II (32.6%)was significantly higher than that in agr non-group II (16%) (P=0.04).
Four isolates were positive in hVISA screening using E-test GRD. Of those, three isolates belonged to agr group II and one isolate belonged to agr group I. All four isolates that were E-test GRD positive had vancomycin MICs of 2 µg/mL measured by the BMD method and the MicroScan panel.
When determining the vancomycin MIC of MRSA isolates, one needs to take into consideration the differences among the methods used for the antibiotic susceptibility test. E-test and MicroScan system estimate higher vancomycin MICs than the Vitek 2 system, which measures it at a lower level than BMD, the reference method [6, 17]. This study showed 100% categorical agreement, as all target isolates were vancomycin susceptible, using both the MicroScan panel and BMD. However, the number of isolates with a MIC of 2 µg/mL was significantly higher when measured using the MicroScan panel than when using BMD.
A study that collected and analyzed isolates of S. aureus from different regions around the world reported that they belong primarily to agr group I [18]. In addition, a study conducted in Germany showed agr group I to be predominantly represented in epidemic MRSA [19]. On the other hand, agr group II is reported to be commonly detected in patients with chronic wounds [20]. Furthermore, a study conducted in a teaching hospital in Korea using MRSA isolated from various clinical specimens showed that 49.3% of isolates belonged to agr group I and 44.0% belonged to agr group II, showing a relatively high percentage of agr group II [21]. This study was conducted by using bloodstream isolates collected from three teaching hospitals in Korea and showed that 50.0% (59/118) of isolates belonged to agr group I and 36.4% (43/118) belonged to agr group II, demonstrating a high percentage of agr group II MRSA in Korea (Table 1). This was consistent with the results of a previous study [21].
Various studies showed an association between agr group and vancomycin susceptibility in MRSA. Some studies reported that MRSA infection associated with agr group II had a higher failure rate of vancomycin therapy [8], and that glycopeptide intermediate S. aureus (GISA) and agr group II are related [9]. On the other hand, some studies reported that reduced susceptibility to glycopeptides is related to both agr group I and II [15] and that there is no association between agr polymorphism and vancomycin resistance [22]. In this study, MRSA associated with agr group II showed a significantly higher percentage of isolates with vancomycin MIC of 2 µg/mL compared with agr non-group II. Considering the association between MRSA with vancomycin MIC of 2 µg/mL and the therapeutic failure of glycopeptides, the present results support those of a previous study that reported an association between agr group II and failure of vancomycin therapy [8]. Several reports suggested that agr dysfunction is associated with worse outcomes among patients with S. aureus infections [10, 23]. This study investigated not the expression of agr genes but the presence of the agr groups.
hVISA is related to the therapeutic failure of vancomycin [24, 25] and shows vancomycin susceptible results with MIC higher than 1 µg/mL [13]. One study that investigated MRSA isolated from the blood showed that hVISA was not detected in MRSA with vancomycin MIC of 1 µg/mL or less, while it was detected in 18.1% of MRSA with vancomycin MICs higher than 1 µg/mL [26]. In this study, hVISA screening test was negative for all MRSA isolates with a vancomycin MIC of 1 µg/mL or less, but was positive for 15.4% (4/26) of isolates with a MIC of 2 µg/mL. As three out of four isolates that tested positive in the hVISA screening test belonged to agr group II, an association between hVISA and agr polymorphism could be inferred. However, this study has limitations. The number of hVISA isolates was small, and determination of population analysis profile was not conducted as a confirmatory test for hVISA.
In summary, agr groups I and II were the major groups represented in MRSA isolates from blood cultures in Korea. The percentage of MRSA isolates with vancomycin MIC of 2 µg/mL was the highest in agr group II (32.6%). All four isolates that tested positive in hVISA screening had vancomycin MIC of 2 µg/mL, and three of the four isolates belonged to agr group II.
References
1. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty third Informational supplement, M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute;2013.
2. Appelbaum PC. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents. 2007; 30:398–408. PMID: 17888634.
3. Zhuo C, Xu YC, Xiao SN, Zhang GY, Zhong NS. Glycopeptide minimum inhibitory concentration creep among meticillin-resistant Staphylococcus aureus from 2006-2011 in China. Int J Antimicrob Agents. 2013; 41:578–581. PMID: 23562222.
4. Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance ofmethicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007; 51:2582–2586. PMID: 17452488.
5. Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patientswith methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008; 52:3315–3320. PMID: 18591266.
6. Swenson JM, Anderson KF, Lonsway DR, Thompson A, McAllister SK, Limbago BM, et al. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. J Clin Microbiol. 2009; 47:2013–2017. PMID: 19420170.
7. Shopsin B, Mathema B, Alcabes P, Said-Salim B, Lina G, Matsuka A, et al. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J Clin Microbiol. 2003; 41:456–459. PMID: 12517893.
8. Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC Jr. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004; 38:1700–1705. PMID: 15227615.
9. Sakoulas G, Eliopoulos GM, Moellering RC Jr, Wennersten C, Venkataraman L, Novick RP, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002; 46:1492–1502. PMID: 11959587.
10. Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, et al. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother. 2011; 55:1082–1087. PMID: 21173172.
11. Falagas ME, Makris GC, Dimopoulos G, Matthaiou DK. Heteroresistance: a concern of increasing clinical significance? Clin Microbiol Infect. 2008; 14:101–104. PMID: 18093235.

12. Khatib R, Jose J, MustaA , Sharma M, Fakih MG, Johnson LB, et al. Relevance of vancomycin-intermediate susceptibility and heteroresistance in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011; 66:1594–1599. PMID: 21525024.
13. Wootton M, Walsh TR, MacGowan AP. Evidence for reduction in breakpoints used to determine vancomycin susceptibility in Staphylococcus aureus. Antimicrob Agents Chemother. 2005; 49:3982–3983. PMID: 16127089.
14. Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991; 29:2240–2244. PMID: 1939577.

15. Verdier I, Reverdy ME, Etienne J, Lina G, Bes M, Vandenesch F. Staphylococcus aureus isolates with reduced susceptibility to glycopeptides belong to accessory gene regulator group I or II. Antimicrob Agents Chemother. 2004; 48:1024–1027. PMID: 14982800.
16. Yusof A, Engelhardt A, Karlsson A, Bylund L, Vidh P, Mills K, et al. Evaluation of a new Etestvancomycin-teicoplanin strip for detection of glycopeptide-intermediate Staphylococcus aureus (GISA), in particular, heterogeneous GISA. J Clin Microbiol. 2008; 46:3042–3047. PMID: 18596146.
17. van Hal SJ, Barbagiannakos T, Jones M, Wehrhahn MC, Mercer J, Chen D, et al. Methicillin-resistant Staphylococcus aureus vancomycin susceptibility testing: methodology correlations, temporal trends and clonal patterns. J Antimicrob Chemother. 2011; 66:2284–2287. PMID: 21750101.
18. van Leeuwen W, van Nieuwenhuizen W, Gijzen C, Verbrugh H, van Belkum A. Population studies of methicillin-resistant and -sensitive Staphylococcus aureus strains reveal a lack of variability in the agr D gene, encoding a staphylococcal autoinducer peptide. J Bacteriol. 2000; 182:5721–5729. PMID: 11004170.
19. Strommenger B, Cuny C, Werner G, Witte W. Obvious lack of association between dynamics of epidemic methicillin-resistant Staphylococcus aureus in central Europe and agr specificity groups. Eur J Clin Microbiol Infect Dis. 2004; 23:15–19. PMID: 14652782.
20. Goerke C, Kümmel M, Dietz K, Wolz C. Evaluation of intraspecies interference due to agr polymorphism in Staphylococcus aureus during infection and colonization. J Infect Dis. 2003; 188:250–256. PMID: 12854080.
21. Yoon HJ, Choi JY, Lee K, Yong D, Kim JM, Song YG. Accessory gene regulator group polymorphisms in methicillin-resistant Staphylococcus aureus: an association with clinical significance. Yonsei Med J. 2007; 48:176–183. PMID: 17461514.
22. Maor Y, Lago L, Zlotkin A, Nitzan Y, Belausov N, Ben-David D, et al. Molecular features of heterogeneous vancomycin-intermediate Staphylococcus aureus strains isolated from bacteremic patients. BMC Microbiol. 2009; 9:189. PMID: 19732456.
23. Fowler VGJr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004; 190:1140–1149. PMID: 15319865.
24. Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004; 38:448–451. PMID: 14727222.
25. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011; 55:405–410. PMID: 21078939.
26. Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, et al. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol. 2009; 47:1640–1644. PMID: 19369444.
Fig. 1
Distribution of vancomycin MICs by methods.
Abbreviations: MIC, minimum inhibitory concentration; BMD, broth microdilution.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download