Dear Editor
An isochromosome of the long arm of chromosome 17, i(17q) has been frequently reported in the blast phase of CML [1], and is also associated with various types of hematological diseases [2]. While it is believed that i(17q) as a sole abnormality is a distinctive clinicopathological entity with a high risk of leukemic progression, a subset may be present as de novo AML [3].
Extreme thrombocytosis is rare in AML, and only a few cases have been described with chromosome 3 abnormalities [4, 5]. In addition, the loss of heterozygosity (LOH) affecting chromosome 7q is common in AML and MDS, suggesting the essential role of this region in disease phenotypes and in clonal evolution. Presented here is a case of AML with myelodysplasia-related changes (AML-MRC) with extreme thrombocytosis, 7q LOH, and i(17q).
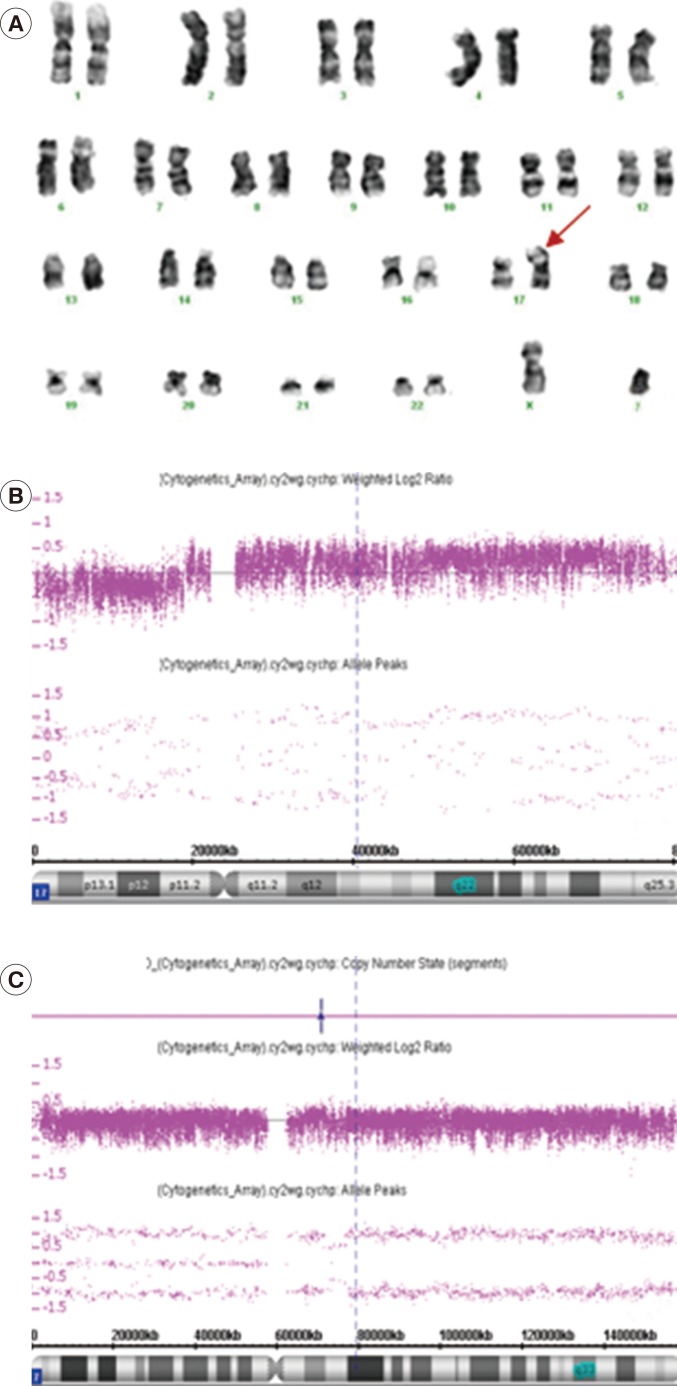
A 76-yr-old Korean male was referred for thrombocytosis. The initial complete blood count (CBC) showed a Hb level of 8.9 g/dL, a platelet count of 1,746×109/L, and a white blood cell (WBC) count of 14.65×109/L with 43% blasts (Fig. 1A). The dysplastic features observed on a peripheral blood smear were marked anisocytosis of red blood cells (RBCs), pseudo-Pelger-Huet-like neutrophils, and extreme thrombocytosis with giant and large platelets (Fig. 1B). Bone marrow aspiration smears displayed hardly visible hematopoietic components owing to extreme thrombocytosis and dyspoietic megakaryocytes (Fig. 1C). Micromegakaryocytes with the dyspoietic features of binucleation or non-lobulated shapes were observed (Fig. 1D). From visible fields located at the periphery of aspiration slides, 37.8% of leukemic blasts were seen. Focal fibrosis of the bone marrow was observed from the biopsy section. The results of BCR-ABL1, JAK2 V617F, MPL W515L/K, and CALR exon 9 mutation tests were all negative. Immunophenotyping revealed that the blasts were positive for CD34, CD13, HLA-DR (moderate), CD33, and CD38 (dim), which was consistent with AML. The chromosome study showed a karyotype of 46,XY,i(17)(q10) in 18 out of 20 metaphase cells (Fig. 2A). The patient was diagnosed as having AML-MRC.
A high-resolution microarray analysis using a cytogenetics whole genome 2.7M array (Affymetrix, Santa Clara, CA, USA) was conducted after obtaining informed consent for further analysis. The microarray analysis also revealed abnormalities of chromosome 17, which was consistent with the conventional cytogenetic findings; the abnormalities were represented as arr 17p13.3p11.2(64,214-18,751,820)×1,17p11.2q25.3 (18,751,820-80,587,411)×3 (Fig. 2B). Incidentally, the microarray analysis also detected a copy neutral LOH as arr7q11.1q36(59,000,001-159,138,663)×2 homozygous (hmz) (Fig. 2C). After being diagnosed as having AML-MRC, the patient refused to continue with chemotherapy and expired 10 months after diagnosis.
According to the study by Rashmi et al. [3], most cases of myeloid neoplasm with i(17q) show anemia, leukocytosis, thrombocytopenia, and splenomegaly. Morphologically, all cases show features of both myelodysplasia and myeloproliferation (pseudo-Pelger-Huet-like neutrophils, micromegakaryocytic hyperplasia, hypercellularity, fibrosis, and osteosclerosis) [3]. It has been suggested that granulocyte colony-stimulating factor and myeloperoxidase positioned at 17q21.1 and 17q23.1, respectively [6, 7], are responsible for myeloproliferative features in the presence of i(17q), where duplication of 17q occurs [3, 8]. In our case, most of the features were consistent with characteristic findings of myeloid neoplasms with i(17q). However, extreme thrombocytosis was a characteristic feature that differed from the previous report, which reported mostly thrombocytopenia [3].
Marked thrombocytosis is rarely associated with AML, and thrombocytosis with a platelet count over 1.0×1012/L is an extremely rare phenomenon, even for a patient with chromosome 3 abnormalities [4]. Our patient had a platelet count of 1,746×109/L at diagnosis, but no abnormalities of chromosome 3 were found. To our knowledge, this is the first reported case of marked thromobocytosis with AML associated with i(17q) and not with chromosome 3.
During further investigation of i(17q) using a single nucleotide polymorphism array (SNP-A), copy neutral LOH 7q was incidentally detected. LOH 7q is common in AML and MDS and seems to play an important role in the phenotype and characteristics of these diseases [9]. A recent study by Jerez et al. [9] found a correlation between the presence of LOH 7q and diploid MDS/MPN. However, no AML-MRC cases had extreme thrombocytosis.
Our case showed features that were consistent with i(17q) in myeloid neoplasms; most of the morphological and clinical features of our patient could be explained as characteristic features of i(17q). However, the finding of marked thromobocytosis and 7q LOH, seemed rather irrelevant and unrelated. A close follow-up of such unusual cases could provide further clinical information on AML-MRC with extreme thrombocytosis accompanied by i(17q) and 7q LOH, while additional studies are necessary to delineate and characterize the development of such unique cases.
Acknowledgements
This research was supported by a grant from Kyung Hee University in 2013 (KHU-20130528).
References
1. Fioretos T, Strömbeck B, Sandberg T, Johansson B, Billström R, Borg A, et al. Isochromosome 17q in blast crisis of chronic myeloid leukemia and in other hematologic malignancies is the result of clustered breakpoints in 17p11 and is not associated with coding TP53 mutations. Blood. 1999; 94:225–232. PMID: 10381517.

2. Mertens F, Johansson B, Mitelman F. Isochromosomes in neoplasia. Genes Chromosomes Cancer. 1994; 10:221–230. PMID: 7522535.

3. Kanagal-Shamanna R, Bueso-Ramos CE, Barkoh B, Lu G, Wang S, Garcia-Manero G, et al. Myeloid neoplasms with isolated isochromosome17q represent a clinicopathologic entity associated with myelodysplastic/ myeloproliferative features, a high risk of leukemic transformation, and wild-type TP53. Cancer. 2012; 118:2879–2888. PMID: 22038701.
4. Lim G, Kim MJ, Oh SH, Cho SY, Lee HJ, Suh JT, et al. Acute myeloid leukemia associated with t(1;3)(p36;q21) and extreme thrombocytosis: a clinical study with literature review. Cancer Genet Cytogenet. 2010; 203:187–192. PMID: 21156232.

5. Hess JL. t(1;3)(p36;q21). Atlas Genet Cytogenet Oncol Haematol. 2002. 5. Last accessed on May 18, 2010. Available at: http://AtlasGeneticsOncology.org/Anomalies/t0103.html.
6. Simmers RN, Smith J, Shannon MF, Wong G, Lopez AF, Baker E, et al. Localization of the human G-CSF gene to the region of a breakpoint in the translocation typical of acute promyelocytic leukemia. Hum Genet. 1988; 78:134–136. PMID: 2448221.

7. Chang KS, Schroeder W, Siciliano MJ, Thompson LH, McCredie K, Beran M, et al. The localization of the human myeloperoxydase gene is in close proximity to the translocation breakpoint in acute promyelocytic leukemia. Leukemia. 1987; 1:458–462. PMID: 2823022.
8. Fiedler W, Weh HJ, Hegawisch-Becker S, Hossfeld DK. GCSF gene is expressed but not rearranged in a patient with isochromosome 17q positive acute nonlymphocytic leukemia. Cancer Genet Cytogenet. 1993; 68:49–51. PMID: 7687196.

9. Jerez A, Sugimoto Y, Makishima H, Verma A, Jankowska AM, Przychodzen B, et al. Loss of heterozygosity in 7q myeloid disorders: clinical associations and genomic pathogenesis. Blood. 2012; 119:6109–6117. PMID: 22553315.

Fig. 1
The findings of the peripheral blood (PB) smear and bone marrow (BM) aspiration. The PB smear shows leukemic blasts (horizontal arrow) (A). Dysplastic features were found on the PB smear such as marked anisopoikilocytosis in red blood cells (RBCs), pseudo-Pelger-Huet-like neutrophils (vertical arrow), and extreme thrombocytosis with giant and large platelets (B). The BM aspirate smear shows that hematopoietic components are barely visible owing to extreme thrombocytosis (C) and micromegakaryocytes display dyspoietic features of binucleation or non-lobulated shapes (D). Wright-Giemsa; ×200 (C), ×1,000 (A, B, D).
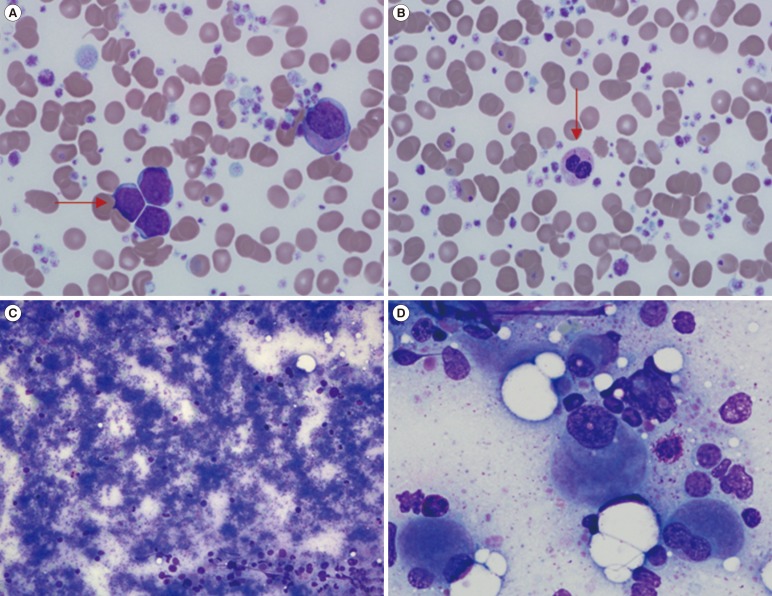
Fig. 2
Chromosome and microarray analyses. Giemsa-banded karyogram of the bone marrow cells at diagnosis: 46,XY,i(17)(q10). The arrow denotes the abnormal chromosome (A). Microarray analysis shows a single copy loss in chromosome 17 at bands p13.3 through p11.2, and a single copy gain of chromosome 17 at bands p11.2 through q25.3 [arr 17p13.3p11.2(64,214-18,751,820)×1, 17p11.2q25.3(18,751,820-80,587,411)×3] (B). SNP array analysis shows homozygosity in the long arm of chromosome 7, at band q11.1, which is approximately 135.9 megabases [arr 7q11.1q36(59,000,001-159,138,663)×2 homozygous (hmz)] (Affymetrix cytogenetics 2.7M array; Affymetrix, Santa Clara, CA, USA) (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download