Abstract
Background
Calreticulin (CALR) mutations were recently discovered in patients with myeloproliferative neoplasms (MPNs). We studied the frequency and type of CALR mutations and their hematological characteristics.
Methods
A total of 168 MPN patients (36 polycythemia vera [PV], 114 essential thrombocythemia [ET], and 18 primary myelofibrosis [PMF] cases) were included in the study. CALR mutation was analyzed by the direct sequencing method.
Results
CALR mutations were detected in 21.9% of ET and 16.7% of PMF patients, which accounted for 58.5% and 33.3% of ET and PMF patients without Janus kinase 2 (JAK2) or myeloproliferative leukemia virus oncogenes (MPL) mutations, respectively. A total of five types of mutation were detected, among which, L367fs*46 (53.6%) and K385fs*47 (35.7%) were found to be the most common. ET patients with CALR mutation had lower leukocyte counts and ages compared with JAK2-mutated ET patients.
The recurrent Janus kinase 2 (JAK2) V617F mutation has been an important molecular marker for myeloproliferative neoplasms (MPNs) since its discovery in 2005 [1]. Polycythemia vera (PV) is almost invariably associated with JAK2 mutations (JAK2 V617F and JAK2 exon 12 mutation), whereas only 50-60% of essential thrombocythemia (ET) and primary myelofibrosis (PMF) cases are associated with the JAK2 V617F mutation. Among the 40% of patients with ET and PMF who lack the JAK2 V617F mutation, 3-5% carry mutations at codon 515 of the gene encoding the thrombopoietin receptor, myeloproliferative leukemia virus oncogene (MPL) [2]. These JAK2 mutations and MPL W515 mutations are gain-of-function mutations that induce overactivation of the signal transducer and transcription (STAT)-signaling activator by autophosphorylation of JAK2. The close associations between JAK2 mutations and MPNs led to the revision of the WHO diagnostic criteria in 2008; these mutations are included in the new major diagnostic criteria for the three classic MPNs.
Other studies reported mutations such as EZH2, ASXL1, TET2, DNMT3A, and IDH1/2 in MPN patients. However, these mutations exist in a small fraction of patients or are detected in other myeloid disorders and not only in MPN. Therefore, as these mutations are not exclusive to JAK2 V617F, they cannot serve as suitable diagnostic markers for classic MPNs [3].
Recently, two independent research groups reported a recurrent calreticulin (CALR) somatic mutation in MPN patients through an exome sequencing approach [4, 5]. Most of these mutations are located in exon 9 and are of insertion/deletion types. Although these mutations are variable, the CALR mutations collectively result in the development of one base pair frameshift and one mutant protein with a novel C terminus. Albeit some rare exceptions, CALR mutations are mutually exclusive to JAK2 mutation or MPL mutation and occur in ET and PMF patients, but not in PV patients. Therefore, CALR mutation was suggested as a powerful diagnostic tool for patients with ET or PMF that are negative for JAK2/MPL mutations.
In this study, we investigated the usefulness of CALR mutations as a diagnostic marker in MPN patients. For this purpose, the frequency and type of CALR mutations as well as the hematological characteristics were analyzed.
The current study was approved by our institutional review board (IRB14-035), and informed consent was obtained from all patients involved. DNA samples that were collected from MPN patients at the time of diagnosis or first referral and then stored were used for this study. The DNA samples were collected from the bone marrow or peripheral blood. A total of 168 MPN patients (85 men, 83 women; mean age 62.8 yr) were included in this study. The patients consist of 36 PV, 114 ET, and 18 PMF. The clinical and laboratory data were reviewed from medical records.
For analysis of CALR mutations, the genomic DNA was extracted from cells by using the QIAamp DNA Mini Kit (QIAGEN Gmbh; Hilden, Germany). Oligonucleotide primers targeting exon 9 of CALR were used to amplify a 483-bp product. The primers were CALR (forward) 5'-CATACGCTGAGGAGTTTGGC-3' and (reverse) 5'-GAGTGGAGGAGGGGAACAAA-3'. DNA was amplified by using the Smart Tag Pre-mix (Solgent Co., Daejeon, Korea). Briefly, 200 ng of DNA template and 1 µL each of forward and reverse primers were added to the PCR premix (20 µL final volume). The amplification parameters included an initial denaturation step at 94℃ for 30 sec, annealing at 63℃ for 30 sec, and extension at 72℃ for 60 sec. The quality and sizes of the PCR products were assessed by electrophoresis on 1.5% agarose gel. Products were purified and bidirectionally sequenced by using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on the ABI 3730XL DNA Analyzer (Applied Biosystems).
The JAK2 V617F and MPL mutations were analyzed by using the JAK2 V617F Kit (Biosewoom, Seoul, Korea) and the Real-Q MPL W515L/K Screening Kit (Biosewoom), respectively. JAK2 exon 12 mutation was analyzed by the direct sequencing method using primers forward 5'-CATACGCTGAGGAGTTTGGC-3'; JAK2 reverse 5'-GAGTGGAGGAGGGGAACAAA-3'.
The differences in the distribution of continuous variables between the categories were analyzed by either the Mann-Whitney test (for nonparametric analysis) or the independent t-test (for parametric analysis). P<0.05 was considered statistically significant. The SPSS 20.0 (SPSS Inc., Chicago, IL, USA) statistical program was used for all calculations.
From the 168 patients tested, CALR mutations were detected in 28 patients (16.7% of all MPNs). The CALR mutational frequencies in each subtype were 0% for PV, 21.9% (25/114) for ET, and 16.7% (3/18) for PMF. The JAK2 mutational frequencies were 86.1% (31/36) for PV, 59.6% (68/114) for ET, and 50.0% (9/18) for PMF (Table 1). JAK2 exon12 mutations were not detected in any patient. MPL mutations were detected only in the ET patients (4.4%; 5/114). For patients with no JAK2 or MPL mutation, the CALR mutational frequency was 58.5% (24/41) for ET and 33.3% (3/9) for PMF. One ET patient had both CALR and JAK2 V617F mutations. The incidence of triple-negative (negative for JAK2/MPL/CALR) patients was 13.9% (5/36) for PV, 14.0% (16/114) for ET, and 33.3% (6/18) for PMF.
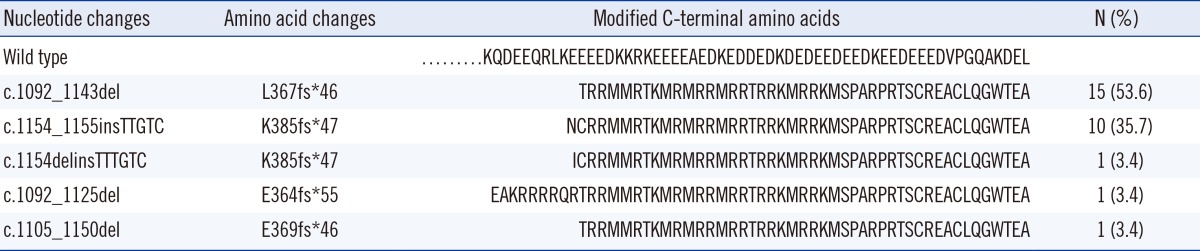
A total of five types of CALR mutations were detected in this study (Fig. 1). Among them, L367fs*46 was the most common type (53.6%; 15/28), followed by K385fs*47 (35.7%; 10/28), E364fs*55 (3.6%; 1/28), and E369fs*46 (3.6%; 1/28). One ET patient harbored another type of K385fs*47 which was combined with an A deletion and a TTTGTC insertion (1154_delins TTTGTC), which was different from common K385fs*47 having a TTGTC insertion (Table 2).
On comparing the hematological parameters between CALR and JAK2 mutants, ET patients with CALR mutations were younger (P=0.025) and had a lower leukocyte count (P<0.001). Hemoglobin and platelet counts were not statistically different between the two groups. In PMF patients, no significant differences were noted in the age and the hemoglobin, leukocyte, and platelet counts (Table 3).
CALR is a highly conserved, multifunctional endoplasmic reticulum (ER) protein. Functionally, CALR plays an integral role in calcium homeostasis and protein folding inside the ER [6]. In addition, outside the ER, CALR regulates integrin-mediated cell adhesion, gene nuclear transport, programmed cell removal, and immunogenic cell death. The C-domain of this protein is involved in calcium regulation and contains a KDEL sequence, which is responsible for preventing the secretion of protein from the ER. CALR encoding CALR protein is located on chromosome 19p13.2, contains 9 exons, and spans a 4.2-kb region [7].
Recently, CALR mutations were reported in ET and PMF patients with non-mutated JAK2/MPL [4, 5]. All CALR mutations reported in MPNs were located on exon 9 and were of the insertion/deletion type. Although the mutations are variable, all mutations cause a frameshift to a unique alternative reading frame. This new reading frame results in the loss of most of the acidic domain, including the KDEL signal, and creates a novel C-terminus protein comprising a minimum of 36 amino acids [4]. This novel sequence contains several positively charged amino acids, whereas the wild-type sequence is mostly negatively charged. Moreover, the exact pathological role of the CALR mutation has not yet been discovered. However, a mutated novel C terminus is suggested to disrupt the ER-signaling peptide and influence the CALR subcellular localization, stability, function, or any combination of these [4].
In MPNs, CALR mutations have been found only with ET and PMF, but not with PV. Despite few reports on cases harboring both JAK2 and CALR mutations [7, 8], in this study, almost all CALR mutations were found in ET/PMF without any JAK2/MPL mutations. In prior reports, the frequency of CALR mutation in JAK2/MPL negative cases was 48.9-82% for ET and 43-88% for PMF [4, 5, 7, 9, 10, 11]. This diverse frequency range can be attributed to the difference in the method of patient selection, sample size, and sensitivity of the detection methods. In studies using whole-genome sequencing method or capillary fragment analysis, the frequency of CALR was visibly greater than that of CALR in other studies that used direct sequencing methods (Table 4). In our study, the direct sequencing method demonstrated CALR mutation in 58.5% and 33.3% of the JAK2/MPL-negative ET and PMF patients, respectively. Its frequency in ET was similar to that reported in prior studies using direct sequencing methods. However, for PMF patients, the frequency of CALR (33.3%) was markedly low, probably owing to the small sample size.
CALR mutation was also detected in other hematological malignancies such as refractory anemia (RA; 9%), RA with ringed sideroblasts (RARS; 11%), and RA with excess blasts (RAEB; 12%). RARS is associated with marked thrombocytosis (RARS-t; 9-12%), chronic myelomonocytic leukemia (CMMoL; 3%), and atypical chronic myelogenous leukemia (aCML; 3%) [4, 5]. However, these diseases have not been studied extensively. In a subsequent study for RARS-t, similar frequencies were not reproduced [12].
As reported in previous studies, several mutational types of CALR have been discovered. Among them, L367fs*46 (52 nucleotide deletion) and K385fs*47 (TTGTC insertion) accounted for >80% of the mutation types. In our study, L367fs*46 and K385fs*47 were detected in 89.3% of both the ET and PMF patients. Other mutation types, including E364fs*55, E369fs*46, and K385fs*47 (TTTGTC insertion), were rarely detected. All five of these mutations also generated a specific C-terminus sequence that included a common peptide sequence (RMRRMR RTRRKMRRKMSPARPRTSCREACLQGWTEA) reported in previous studies (Table 2). This commonly mutated sequence could be a target for diagnostic methods or therapeutic approaches. In a recent study, immunostaining was performed by using an antibody specific for the mutated amino acids of CALR. The researchers revealed the preferential expression of megakaryocytes for CALR and recommended immunostaining as a complementary method for molecular analysis [13].
The effect of CALR mutation on the hematological phenotype has been reported in several studies. Compared with ET patients with JAK2 mutation, those with CALR mutation had lower leukocyte counts and hemoglobin values and higher platelet counts [4, 9, 10]. PMF patients with CALR mutations also showed similar results to those of CALR mutation associated with a higher platelet count and lower leukocyte count [4, 7]. In our study, a significant association with CALR mutations was noted only in ET patients with a lower leukocyte count. The platelet count seemed to be higher in CALR-mutated ET patients than in those with JAK2 mutation, although the statistical significance was not deduced. Among PMF patients, no CALR-associated phenotype was detected, possibly because of the small sample size.
Although the impact of CALR mutation on the thrombosis risk or the clinical outcome was not analyzed in this study, it has been reported in previous studies. Rumi et al. [10] reported that ET patients with CALR mutation had a lower polycythemic transformation rate, but not a lower myelofibrotic transformation rate, compared with ET patients with JAK2 mutation. Klampfl et al. [4] also demonstrated a low risk of thrombosis and longer survival for CALR-positive ET and PMF patients. However, a neutral effect has also been reported. For instance, Rotunno et al. [9] reported that CALR mutation had no impact on the survival or transformation to post-ET myelofibrosis. Interestingly, Tefferi et al. [14] attempted molecular prognostication for PMF patients based on a combination of CALR and additional sex combs like 1 (ASXL1) modifications. They found that CALR(-)/ASXL1(+) was a powerful risk factor representing poor prognosis. Further evaluation is required for asserting the usefulness of additional ASXL1 genotyping for PMF.
In conclusion, CALR mutation is a useful diagnostic marker for JAK2/MPL-negative ET or PMF patients because of its mutual exclusiveness for JAK2 mutation and the relative high frequency. The phenotypic manifestations of CALR mutation are different from those of JAK2 mutation, and this fact may facilitate defining subtypes with different prognoses.
Acknowledgments
We thank KS Park, KS Lee, and TY Park for their helpful assistance with sample collection, DNA extraction, and sequencing analysis.
References
1. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–1790. PMID: 15858187.
2. Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006; 3:e270. PMID: 16834459.

3. Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010; 24:1128–1138. PMID: 20428194.

4. Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013; 369:2379–2390. PMID: 24325356.

5. Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013; 369:2391–2405. PMID: 24325359.
6. Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997; 386:843–847. PMID: 9126744.

7. Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014; 28:1472–1477. PMID: 24402162.

8. Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014; 123:2220–2228. PMID: 24478400.

9. Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014; 123:1552–1555. PMID: 24371211.

10. Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014; 123:1544–1551. PMID: 24366362.

11. Li B, Xu J, Wang J, Gale RP, Xu Z, Cui Y, et al. Calreticulin mutations in Chinese with primary myelofibrosis. Haematologica. 2014; pii: haematol.2014.109249. [Epub ahead of print].

12. Patnaik MM, Belachew A, Finke C, Lasho TL, Hanson CA, Tefferi A. CALR mutations are infrequent in WHO-defined refractory anemia with ring sideroblasts. Leukemia. 2014; 28:1370–1371. PMID: 24476767.

13. Vannucchi AM, Rotunno G, Bartalucci N, Raugei G, Carrai V, Balliu M, et al. Calreticulin mutation-specific immunostaining in myeloproliferative neoplasms: pathogenetic insight and diagnostic value. Leukemia. 2014; 28:1811–1818. PMID: 24618731.

14. Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014; 28:1494–1500. PMID: 24496303.

Fig. 1
The five types of calreticulin mutations identified in this study. The arrowheads indicate the points where insertion or deletion occurred. Square boxes represent nucleotide sequences that were deleted or inserted.
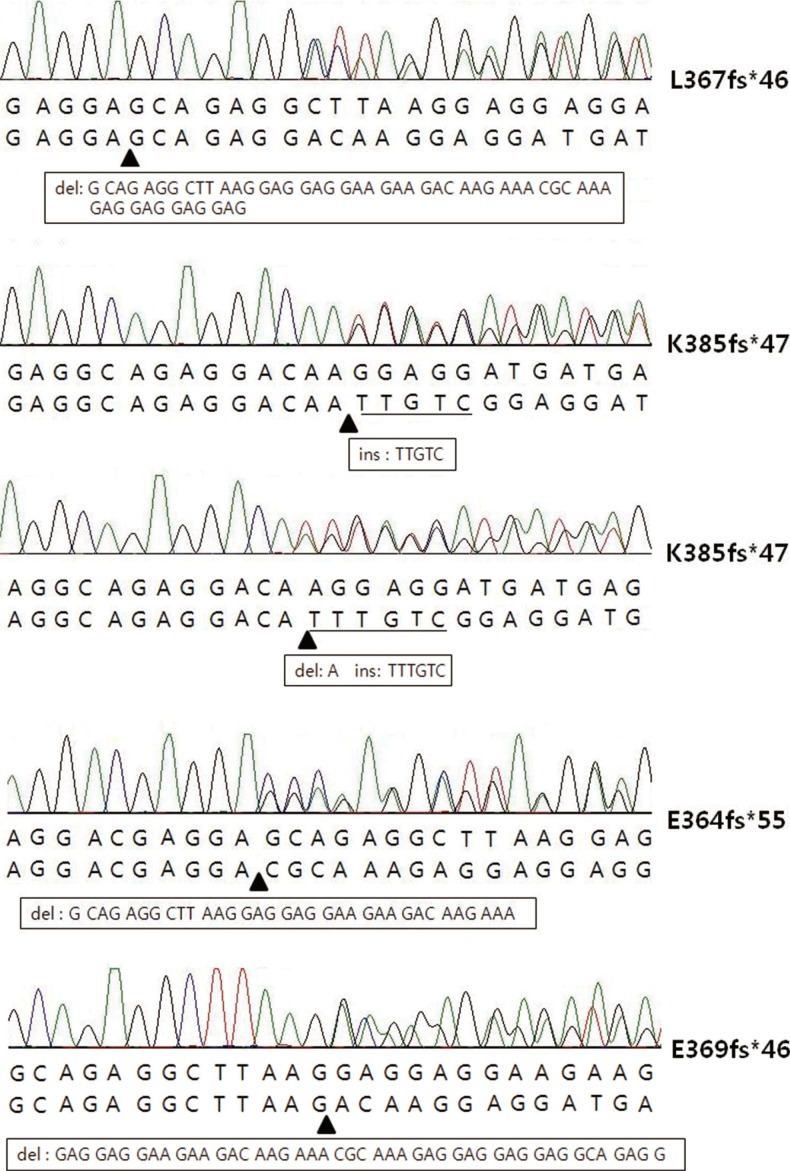
Table 1
The frequency of JAK2 V617F, MPL W515, and CALR mutations
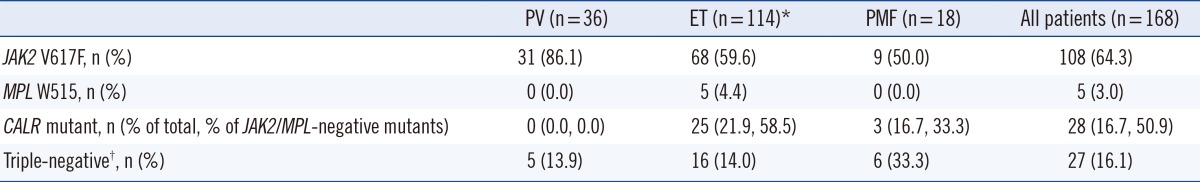
*One ET patient had both CALR and JAK2 V617F mutations; †Negative for JAK2 (V617F and exon 12 mutation), MPL W515, and CALR mutation.
Abbreviations: JAK2, Janus kinase 2; MPL, myeloproliferative leukemia virus oncogene; CALR, calreticulin; PV, polycythemia vera; ET, essential thrombocythemia; PMF, primary myelofibrosis.
Table 3
Hematological characteristics of CALR mutant and JAK2 V617F mutant in ET and PMF patients
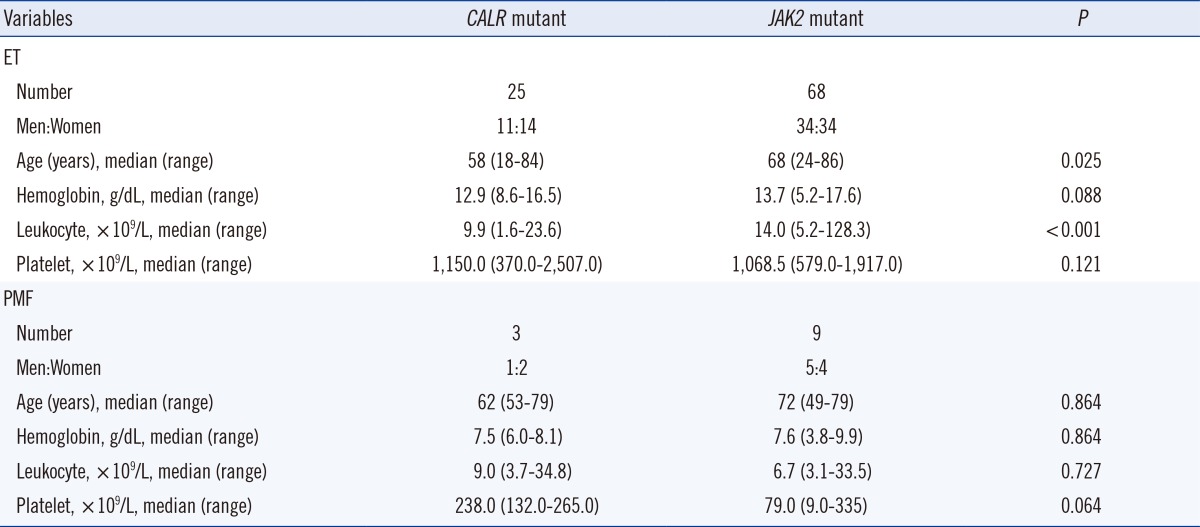
Abbreviations: see Table 1.
Table 4
The frequencies of CALR mutation reported by previous studies
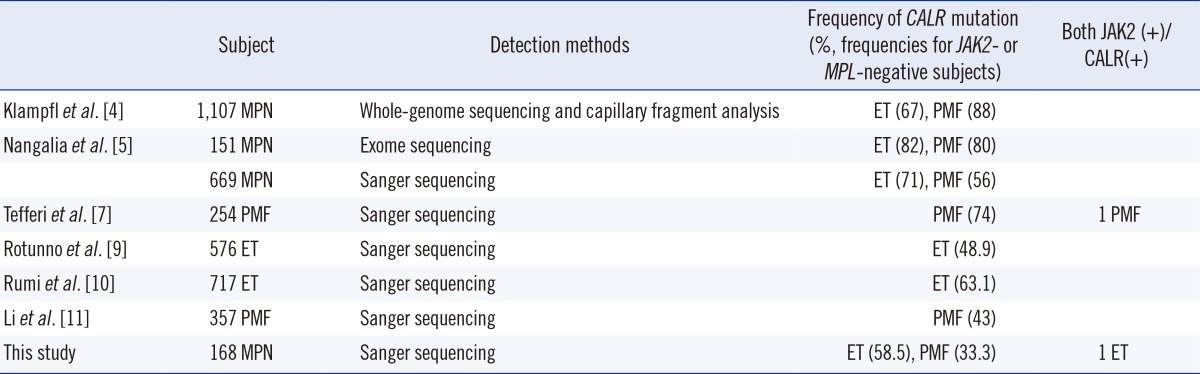
Abbreviations: MPN, myeloproliferative neoplasms; JAK2, MPL, CALR, ET, PMF; see Table 1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download