Abstract
Background
Bone marrow biopsies are routinely performed for staging patients with B-cell non-Hodgkin lymphoma (NHL). In addition to histomorphological studies, ancillary tools may be needed for accurate diagnosis. We investigated the clinical utility of multiparameter flow cytometric examination of bone marrow aspirates.
Methods
A total of 248 bone marrow specimens from 232 patients diagnosed with B-cell NHL were examined. Monoclonal antibodies directed against CD19, CD20, CD10 (or CD5), and κ and λ immunoglobulins were used. Multi-stage sequential gating was performed to select specific cells of interest, and the results were compared with bone marrow histology.
Results
The concordance rate between histomorphology and flow cytometry was 91.5% (n=227). Eight cases (3.2%) were detected by flow cytometry alone and were missed by histomorphology analysis, and 6 of these 8 cases showed minimal bone marrow involvement (0.09-2.2%). The diagnosis in these cases included large cell lymphoma (n=3), mantle cell lymphoma (n=3), and mucosa-associated lymphoid tissue (MALT) lymphoma (n=2). Thirteen cases were histopathologically positive and immunophenotypically negative, and the diagnoses in these cases included diffuse large cell lymphoma (n=7), T-cell/histiocyte-rich large B-cell lymphoma (n=2), anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (n=1), follicular lymphoma (n=1), MALT lymphoma (n=1), and unclassifiable lymphoma (n=1).
Complete and accurate staging of non-Hodgkin lymphoma (NHL) is essential in determining the extent of disease, which may affect both the prognosis and treatment strategies [1, 2, 3, 4, 5]. Although there has been a continual growth in the number of ancillary tools that can be used in the laboratory to evaluate malignant lymphoma over the last decade [6, 7, 8, 9], evaluation of bone marrow involvement of malignant lymphoma is still an important aspect, and bilateral trephine biopsies have been regarded as the standard method [10].
The utility of flow cytometric analysis in the routine staging of NHL has been evaluated by several previous studies [11, 12, 13, 14, 15, 16, 17, 18, 19, 20]; however, sufficient data and standardization of protocols are lacking. Moreover, with the advance of technology, the usefulness of a multi-color strategy for diagnosis has been increasingly recognized, but has not been fully evaluated. For this reason, we evaluated the roles of six-color multiparameter flow cytometric analysis of bone marrow aspirate in the staging of B-cell NHL in the Korean patient population.
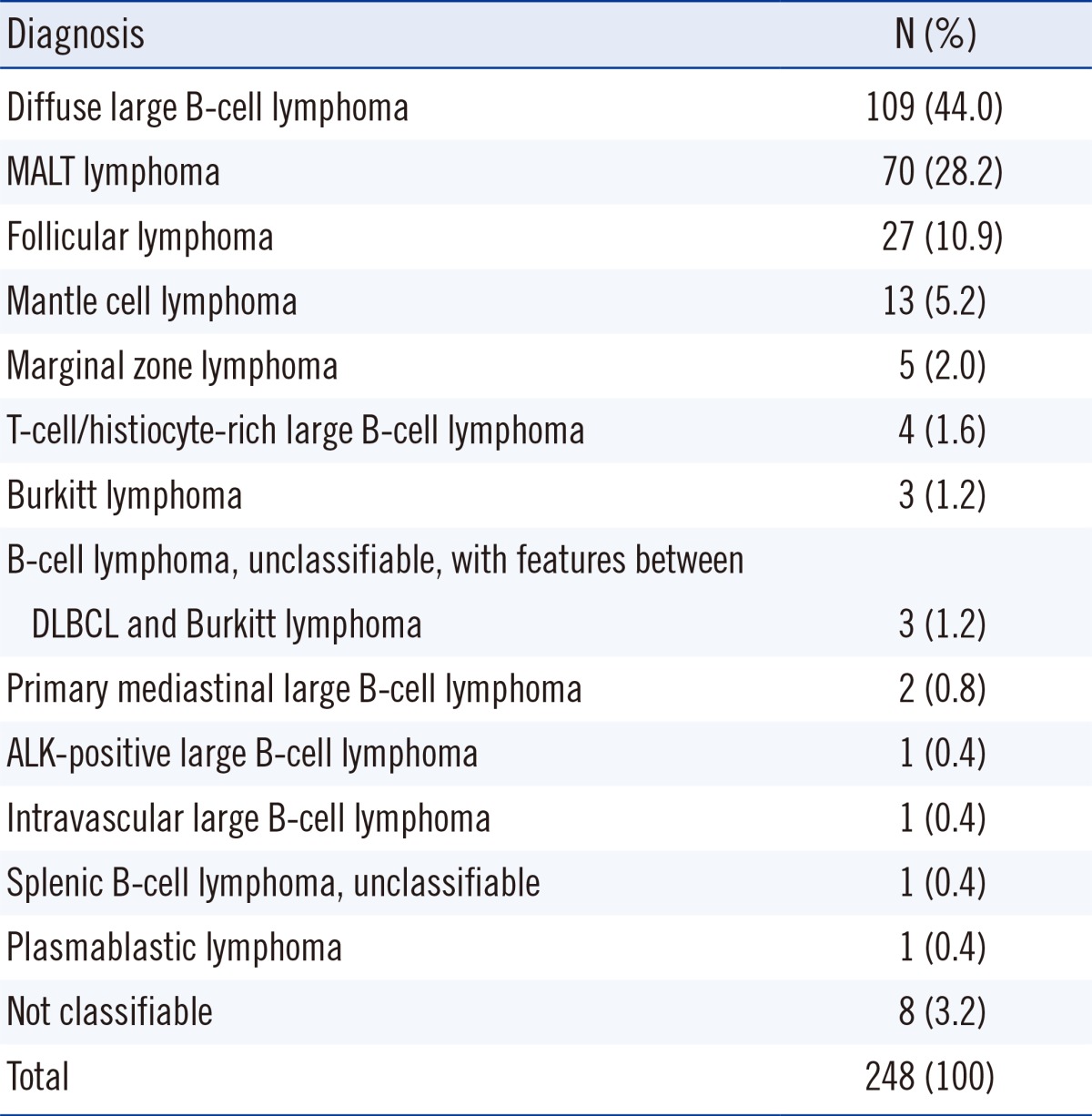
We collected 248 bone marrow specimens from 232 patients (137 men and 95 women) who were diagnosed as having B-cell malignancy between December 2012 and July 2013 at our center: 198 at diagnosis and 50 during the course of the disease. Regarding diagnoses, diffuse large B-cell lymphoma was most common (44.0%), followed by mucosa-associated lymphoid tissue (MALT) lymphoma (28.2%) and follicular lymphoma (10.9%) (Table 1).
Wright-Giemsa-stained slides of bilateral bone marrow aspirate smears were examined for atypical lymphoid/lymphoma cells independent of immunophenotypic studies. Of 248 bone marrow aspirate specimens, five had no cellular component and were excluded from the study. Bilateral bone marrow trephine biopsies were obtained and tissue samples were fixed in 10% neutral-buffered formalin, decalcified, and paraffin-embedded. Hematoxylin and eosin (H&E) staining and CD3 and CD20 immunostaining were performed to determine lymphoma involvement.
Flow cytometric immunophenotyping of an EDTA anticoagulated bone marrow aspirate specimen was performed in each case. A standard bone marrow assay with erythrocyte cell lysis was used for all bone marrow aspirate specimens. Aspirate specimens from one side or a mixture of both right and left sides were used depending on the quality and amount of specimens. The number of cells analyzed was between 50,000 and 250,000, and the instrumental sensitivity was 0.1%.
Samples were analyzed with a two-step protocol; screening was done first with six-color multiparameter flow cytometry to detect the abnormal lymphoid cell population followed by second-line, detailed immunophenotyping for specific characterization of lymphoma cells. For the first step, an analysis with six markers for B-cell lymphoma was performed with monoclonal antibodies directed against CD19, CD20, CD10, and κ and λ immunoglobulins (Igs). In the case of mantle cell lymphoma, a monoclonal antibody against CD5 was used instead of CD10. These antibodies were combined as κ/λ-fluorescein isothiocyanate/lambda-phycoerythrin (PE), CD19-peridinin chlorophyll (PerCP), CD10-allophycocyanin (APC), CD20-PE-cyanine7 (Cy7), and CD45-APC-Cy7. In the case of mantle cell lymphoma, CD5-PerCP and CD19-APC were used. Antibodies were supplied by Becton Dickinson immunocytometry systems (Becton Dickinson, San Jose, CA, USA) except for CD5, which was supplied by Beckman Coulter (Beckman Coulter, Miami, FL, USA). Because lymphomas associated with these cases were classified on the basis of lymph node or tissue biopsies, the first bone marrow immunophenotypic analyses were solely centered on involvement of malignant lymphoma, i.e., the presence of a monoclonal B-cell population. Cutoffs of κ-to-λ ratios have been pre-established as 0.25:1 and 4:1 in our laboratory with normal bone marrow specimens.
The second-line flow cytometric analysis was performed with positive cases in the initial clonality test. This step is aimed at evaluating the detailed immunophenotype of lymphoma cells. A selected panel of antibodies was used and combined as the following: FMC (developed at Flinders Medical Centre) 7/CD23/CD10/CD45, CD5/CD20/CD22/CD45, CD38/CD3/CD56/CD45, and terminal deoxynucleotidyltransferase (TdT). Both forward/side light scatter and CD45/side light scatter were used in each case as primary gating methodologies. Further gating was performed as necessary on either lymphoid subpopulations based on cell size or backgating in the event of CD19+ B-cell staining.
Approximately 24.6% of bone marrow evaluations showed evidence of NHL; bone marrow trephine biopsies showed histologic involvement of lymphoma cells in 50 cases (20.2%) and flow cytometric analysis of bone marrow aspirate showed the presence of lymphoma cells in 45 cases (18.1%).
Forty-five cases (18.1%) showed bone marrow involvement by NHL by flow cytometry, with monoclonal B-cell populations ranging from 0.09% to 60.00% (median 5.12%) of total cell populations. Among the positive cases, 19 (42.2%) showed minimal bone marrow involvement (malignant cells less than 5%; Fig. 1). The 203 cases with a negative flow cytometric study typically showed a mixture of polyclonal B cells and a small population of CD19+/Ig- cells representing normal CD19+ precursor B cells. In these cases, the κ-to-λ ratios of polyclonal B cells ranged from 0.98 to 1.85, and the median was 1.35, which was concordant with previously established normal ranges.
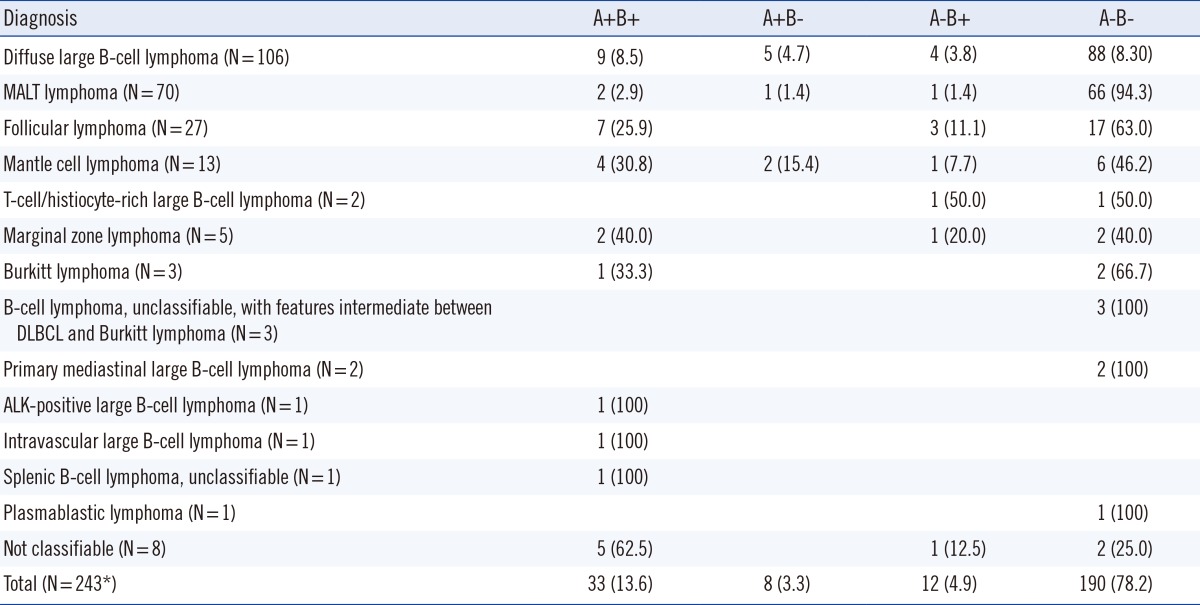
The concordance rate between flow cytometry and trephine biopsy histopathology was 91.5%; both were negative in 190 cases (76.6%) and both were positive in 37 cases (14.9%). Discrepant results were found in 21 cases (8.5%) (Table 2). There were eight cases with histomorphologically negative and immunophenotypically positive results, and the diagnosis in these cases included diffuse large B-cell lymphoma (n=3), mantle cell lymphoma (n=3), and MALT lymphoma (n=2). Of those eight cases, six showed minimal bone marrow involvement (0.09-2.2%). Thirteen cases were histopathologically positive and immunophenotypically negative, and the diagnoses in these cases included diffuse large cell lymphoma (n=7), T-cell/histiocyte-rich large B-cell lymphoma (n=2), anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (n=1), follicular lymphoma (n=1), MALT lymphoma (n=1), and unclassifiable lymphoma (n=1).
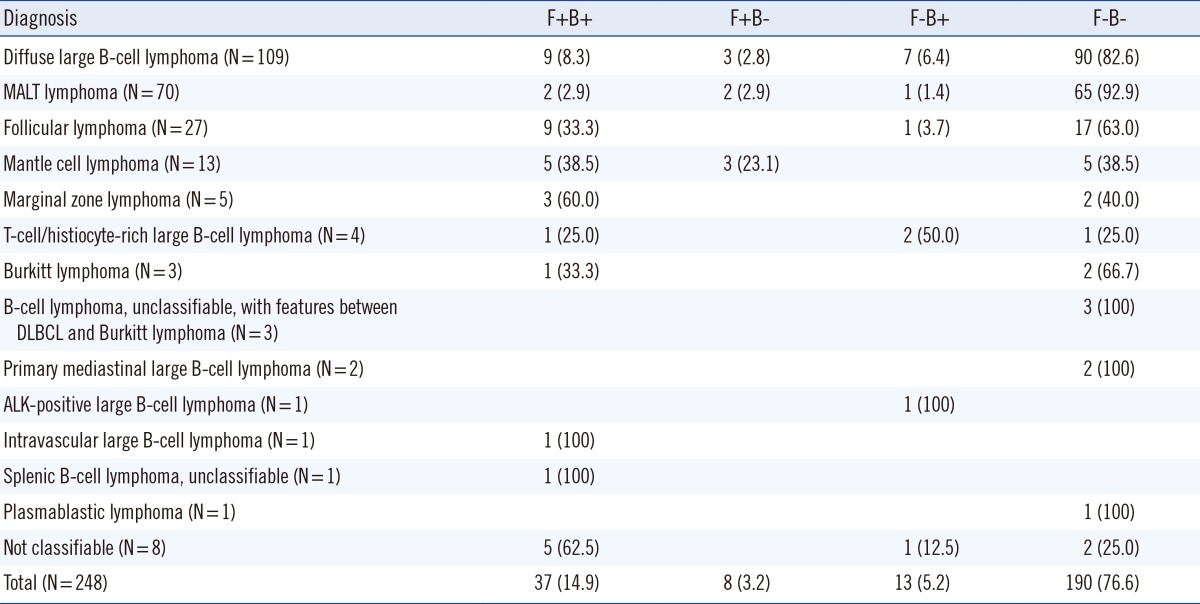
Morpholgic examination and analysis of immunostaining of both bone marrow biopsy and aspirate showed 91.8% concordance rate; both were negative in 190 cases (78.2%) and both were positive in 33 cases (13.6%) (Table 3).
Complete and accurate staging of malignant lymphoma is essential in providing adequate management and predicting outcomes. As a part of lymphoma staging, the evaluation of bone marrow involvement is an important process, and morphological assessment of bone marrow biopsy specimens remains the standard method. Recently, flow cytometric analysis of bone marrow aspirate has been performed as a routine procedure. Although flow cytometry provides more objective results with higher sensitivity, previous studies did not support a significant benefit beyond morphological examination alone [18, 19, 20]. However, the low detection rate by flow cytometry in those studies may be partly related to the use of less sensitive and less specific single- or dual-color analyses, which were the standard at that time, compared with the four- or six-color multiparameter analysis technology that is currently used. With the advanced technology currently available, six-color or eight-color multiparameter analysis can improve the sensitivity and specificity [21, 22]. At this point, evaluation of the utility of flow cytometric analysis on bone marrow using multiparameter analysis could be helpful. We performed both morphological examination of bone marrow biopsy specimens and flow cytometric analysis of bone marrow aspirates concurrently, and compared the results.
The immunophenotyping in this study was performed with six-color multiparameter flow cytometry, which has advantages compared to examination with only CD19, κ, and λ light chains. Including CD10 enabled increased detection sensitivity by excluding normal hematogones, and the inclusion of both CD19 and CD20 was additionally helpful for a more specific selection of B-cell populations according to differentiation status. Moreover, the specificity may be improved by using CD10 in follicular lymphoma and by using CD5 in mantle cell lymphoma. This will result in an improved sensitivity by lowering the possibility of masking small portions of malignant cells by the presence of many polyclonal cells.
The flow cytometric analysis is based on the clonality test. Detection of immunoglobulin light chain-restriction expression represents the most reliable evidence for the presence of malignant cells. In this study, κ-to-λ ratio ranged from 0.98 to 1.85 and the median was 1.35, confirming the normal range previously determined. The second-line, detailed immunophenotyping to detect abnormal immunophenotypes of malignant B-cell populations may increase the sensitivity when the role of screening immunophenotyping is limited, as in cases of malignant B cells with no light chain expression. Detailed immunophenotyping can also provide complementary information, which may be helpful in the accurate subclassification of NHL [23, 24]. This is very useful in cases without easily accessible lymph nodes or in cases with no definite lymphadenopathy.
There were 21 discrepant cases between the results of the bone marrow biopsy and flow cytometric analysis. Among those, eight cases were reported, in which lymphoma cells were not detected by morphological examination. The discrepancy could be explained in part by a low sensitivity of morphological examination in detecting minimal bone marrow involvement. Indeed, six of eight cases showed minimal bone marrow involvement of malignant lymphoma. In 13 cases, however, the involvement of the lymphoma was detected only in the biopsy specimen. This discrepancy may be attributed to differences in the specimens analyzed. For example, when the disease does not diffusely involve the bone marrow and the lymphoid infiltration foci are small, the bone marrow may produce aspirate samples free of disease while the biopsy may be positive for lymphoma cells. Evidence for this is the improved sensitivity of bilateral bone marrow biopsy over unilateral biopsy. The same principle can be applied in the cases, in which lymphoma involvement was not detectable on bone marrow aspirate despite positive results on bone marrow biopsies. The presence of these cases confirms previously published results, which concluded that there is no substitute for a bone marrow biopsy [13, 14].
Additionally, collection of bone marrow aspirate is a simple and minimally invasive procedure that is considered an ancillary tool in the staging of NHL. The utility of collecting bone marrow aspirate has been advocated by some groups [25, 26], while others have not supported the utility of this procedure [27]. To evaluate the usefulness of this specimen, we simultaneously compared bone marrow aspirate and biopsy samples. When aspirate was compared with biopsy specimens by morphological examination and immunostaining, the concordance rate was 91.8% with 20 discrepant cases. On morphological examination of aspirate specimens, it was difficult to confirm the involvement of NHL when a minimal number of lymphoma cells were present or the specimen was diluted with peripheral blood. The utility of flow cytometric examination could be maximized when the cytomorphological findings are equivocal.
We conclude that multi-color flow cytometry and examination of bone marrow aspirate are useful methods for assessing bone marrow in NHL staging. Although they are not a substitute for bone marrow biopsy, they do have a complementary role in detecting a small portion of lymphoma cells in a small subset of patients. Particularly, multi-color flow cytometric analysis can increase the sensitivity of this method, enabling more specific gating of abnormal cell populations. It may also be useful in cases where the morphological evaluation of the bone marrow biopsy is inconclusive or unavailable. Since the clinical significance of such a minimal bone marrow involvement is not fully established, further clinical studies are needed to elucidate the effect of minimal bone marrow involvement on the clinical course and prognosis of patients.
References
1. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329:987–994. PMID: 8141877.
2. Bastion Y, Coiffier B. Is the International Prognostic Index for Aggressive Lymphoma patients useful for follicular lymphoma patients? J Clin Oncol. 1994; 12:1340–1342. PMID: 8021723.

3. Coiffier B, Gisselbrecht C, Vose JM, Tilly H, Herbrecht R, Bosly A, et al. Prognostic factors in aggressive malignant lymphomas: description and validation of a prognostic index that could identify patients requiring a more intensive therapy. The Groupe d'Etudes des Lymphomes Agressifs. J Clin Oncol. 1991; 9:211–219. PMID: 1703226.

4. Munck JN, Dhermain F, Koscielny S, Girinsky T, Carde P, Bosq J, et al. Alternating chemotherapy and radiotherapy for limited-stage intermediate and high-grade non-Hodgkin's lymphomas: long-term results for 96 patients with tumors > 5 cm. Ann Oncol. 1996; 7:925–931. PMID: 9006743.
5. Shipp MA, Neuberg D, Janicek M, Canellos GP, Shulman LN. High-dose CHOP as initial therapy for patients with poor-prognosis aggressive non-Hodgkin's lymphoma: a dose-finding pilot study. J Clin Oncol. 1995; 13:2916–2923. PMID: 8523055.

6. Coad JE, Olson DJ, Christensen DR, Lander TA, Chibbar R, McGlennen RC, et al. Correlation of PCR-detected clonal gene rearrangements with bone marrow morphology in patients with B-lineage lymphomas. Am J Surg Pathol. 1997; 21:1047–1056. PMID: 9298881.

7. Murphy TD, Crisan D, Farkas DH. Use of molecular diagnostic methods for lymphoma staging in bilateral bone marrow biopsies. Mol Diagn. 1997; 2:177–182. PMID: 10462607.

8. Nedomova R, Papajik T, Prochazka V, Indrak K, Jarosova M. Cytogenetics and molecular cytogenetics in diffuse large B-cell lymphoma (DLBCL). Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013; 157:239–247. PMID: 23132512.

9. Reichard KK, Robinett S. Detection of genetic translocations in lymphoma using fluorescence in situ hybridization. Methods Mol Biol. 2013; 999:189–202. PMID: 23666698.

10. Juneja SK, Wolf MM, Cooper IA. Value of bilateral bone marrow biopsy specimens in non-Hodgkin's lymphoma. J Clin Pathol. 1990; 43:630–632. PMID: 2401730.

11. Liu W, Medeiros LJ, Lin P, Romaguera JE, Wang SA, Jorgensen JL. Usefulness of flow cytometrici mmunophenotyping for bone marrow staging in patients with mantle cell lymphoma after therapy. Am J Clin Pathol. 2012; 137:634–640. PMID: 22431541.
12. Merli M, Arcaini L, Boveri E, Rattotti S, Picone C, Passamonti F, et al. Assessment of bone marrow involvement in non-Hodgkin's lymphomas: comparison between histology and flow cytometry. Eur J Haematol. 2010; 85:405–415. PMID: 20662897.

13. Duggan PR, Easton D, Luider J, Auer IA. Bone marrow staging of patients with non-Hodgkin lymphoma by flow cytometry: correlation with morphology. Cancer. 2000; 88:894–899. PMID: 10679660.
14. Hanson CA, Kurtin PJ, Katzmann JA, Hoyer JD, Li CY, Hodnefield JM, et al. Immunophenotypic analysis of peripheral blood and bone marrow in the staging of B-cell malignant lymphoma. Blood. 1999; 94:3889–3896. PMID: 10572105.

15. Morice WG, Kurtin PJ, Hodnefield JM, Shanafelt TD, Hoyer JD, Remstein ED, et al. Predictive value of blood and bone marrow flow cytometry in B-cell lymphoma classification: comparative analysis of flow cytometry and tissue biopsy in 252 patients. Mayo Clin Proc. 2008; 83:776–785. PMID: 18613994.

16. Palacio C, Acebedo G, Navarrete M, Ruiz-Marcellán C, Sanchez C, Blanco A, et al. Flow cytometry in the bone marrow evaluation of follicular and diffuse large B-cell lymphomas. Haematologica. 2001; 86:934–940. PMID: 11532621.
17. Perea G, Altés A, Bellido M, Aventín A, Bordes R, Ayats R, et al. Clinical utility of bone marrow flow cytometry in B-cell non-Hodgkin lymphomas (B-NHL). Histopathology. 2004; 45:268–274. PMID: 15330805.

18. Dunphy CH. Combining morphology and flow cytometric immunophenotyping to evaluate bone marrow specimens for B-cell malignant neoplasms. Am J Clin Pathol. 1998; 109:625–630. PMID: 9576583.

19. Iancu D, Hao S, Lin P, Anderson SK, Jorgensen JL, McLaughlin P, et al. Follicular lymphoma in staging bone marrow specimens: correlation of histologic findings with the results of flow cytometry immunophenotypic analysis. Arch Pathol Lab Med. 2007; 131:282–287. PMID: 17284114.

20. Naughton MJ, Hess JL, Zutter MM, Bartlett NL. Bone marrow staging in patients with non-Hodgkin's lymphoma: is flow cytometry a useful test? Cancer. 1998; 82:1154–1159. PMID: 9506363.
21. Carulli G, Marini A. Diagnosis and classification of B-cell non-Hodgkin lymphomas. The role of multiparameter flow cytometry. Clin Ter. 2012; 163:47–57. PMID: 22362234.
22. Stacchini A, Aliberti S, Demurtas A, Benevolo G, Godio L. Ten antibodies, six colors, twelve parameters: a multiparameter flow cytometric approach to evaluate leptomeningeal disease in B-cell non-Hodgkin's lymphomas. Cytometry B Clin Cytom. 2012; 82:139–144. PMID: 22328059.

23. Fineberg S, Marsh E, Alfonso F, Espiritu E, Gottesman SR, Amorosi E, et al. Immunophenotypic evaluation of the bone marrow in non-Hodgkin's lymphoma. Hum Pathol. 1993; 24:636–642. PMID: 8505041.

24. Jennings CD, Foon KA. Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood. 1997; 90:2863–2892. PMID: 9376567.

25. Barekman CL, Fair KP, Cotelingam JD. Comparative utility of diagnostic bone-marrow components: a 10-year study. Am J Hematol. 1997; 56:37–41. PMID: 9298866.

26. Lee SH, Erber WN, Porwit A, Tomonaga M, Peterson LC. International Council for Standardization In Hematology. ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol. 2008; 30:349–364. PMID: 18822060.

27. Winfield DA, Polacarz SV. Bone marrow histology. 3: Value of bone marrow core biopsy in acute leukaemia, myelodysplastic syndromes, and chronic myeloid leukaemia. J Clin Pathol. 1992; 45:855–859. PMID: 1430254.

Fig. 1
Examples of κ-to-λ ratios for patients with B-cell non-Hodgkin lymphoma using different gating strategies. (A) Plots showing unequivocal light chain restriction. (B) Increased sensitivity using multi-color antibodies. Gating on CD19-positive and CD20-positive and CD10-negative cells could identify very small amounts of clonal B-cells (κ-to-λ ratio=11.5) (C) whereas gating without CD10 masks the clonal B-cell population (κ-to-λ ratio=1.412). (D) Plots showing negative controls.
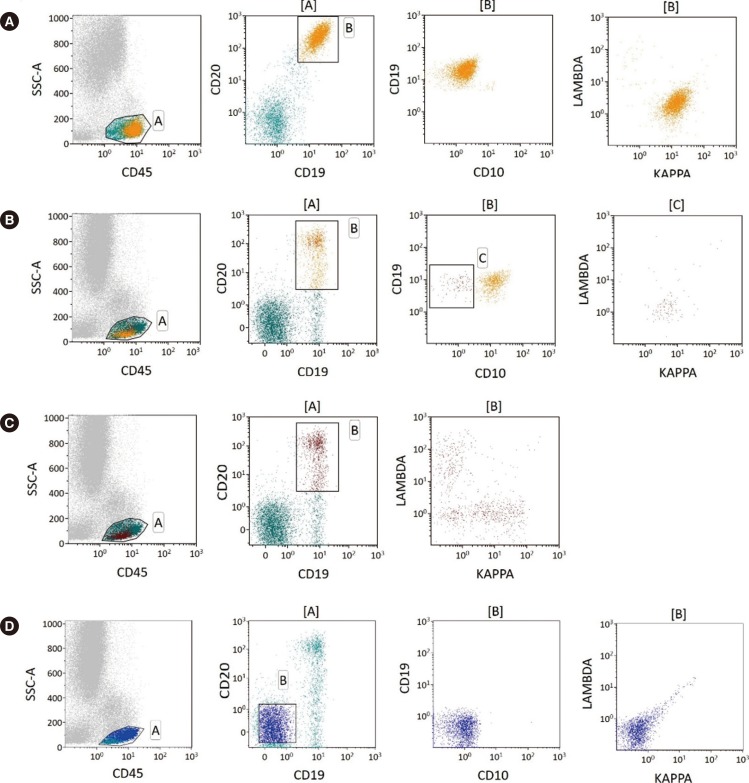
Table 3
B-cell NHL involvement by bone marrow aspirate (A) and core biopsy (B)
Data is presented as number (percentage).
*Among 248 specimens, five bone marrow aspirate specimens were inadequate in quality and excluded from the study (3 with diffuse large B-cell lymphoma and 2 with T-cell/histiocyte-rich large B-cell lymphoma).
Abbreviations: See Table 2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download